Abstract
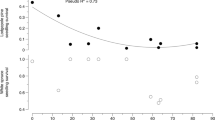
The spatial structure of the environment is known to affect ecological processes. Unlike the spatial structure of negative interactions, such as competition and predation, the role of spatial structure in positive interaction has received less attention. We tested how the spatial structure of spores of ectomycorrhizal fungi (EMF) in the soil affects the growth of Aleppo pine (Pinus halepensis) seedlings. Spores were spatially distributed at four different levels of patchiness (1 patch, 4 patches, 8 patches and complete mixing) in 4 L pots (all pots received the same total amount of spores). Based on previous findings, we hypothesized that plant performance would gradually increase from the single patch treatment to the complete mixing. However, we found a non-linear response to patchiness. Specifically, plants were largest in the single patch and complete mixing while those in the 4 and 8 patch treatments were the smallest. This non-monotonic response, which might be the result of spatially determined colonization timing or community composition, suggests that the spatial structure of EMF spores has a complex effect on seedling growth.




Similar content being viewed by others
Data availability
Sequences were submitted to the National Center for Biotechnology Information Sequence Read Archive with the accession codes: Bioproject PRJNA699879.
References
Adler PB, HilleRisLambers J, Kyriakidis PC, Guan Q, Levine JM (2006) Climate variability has a stabilizing effect on the coexistence of prairie grasses. Proc Natl Acad Sci USA 103:12793–12798
Álvarez-López V, Zappelini C, Durand A, Chalot M (2020) Pioneer tress of Betula pendula at a red gypsum landfill harbour specific structure and composition of root-associated microbial communities. Sci Total Envir:138530
Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M (2009) Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol Lett 12:13–21
Botnen S, Mundra S, Kauserud H, Eidesen P (2020) Glacier retreat in the High Arctic: Opportunity or threat for ectomycorrhizal diversity? FEMS Microbiol Ecol 96:fiaa171
Briggs CJ, Hoopes MF (2004) Stabilizing effects in spatial parasitoid–host and predator–prey models: a review. Theor Popul Biol 65:299–315
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat methods 13:581–583
Chagnon PL, Bradley RL, Klironomos JN (2020) Mycorrhizal network assembly in a community context: the presence of neighbours matters. J Ecol 108:366–377
Chesson P (1996) Matters of scale in the dynamics of populations and communities. Frontiers of Population Ecology pp 353–368
Chesson P (2000) General theory of competitive coexistence in spatially-varying environments. Theor Popul Biol 58:211–237
Cresswell JE (1997) Spatial heterogeneity, pollinator behaviour and pollinator-mediated gene flow: bumblebee movements in variously aggregated rows of oil-seed rape. Oikos pp 546–556
Curtis PG, Slay CM, Harris NL, Tyukavina A, Hansen MC (2018) Classifying drivers of global forest loss. Science 361:1108–1111
Dickie IA, Xu B, Koide RT (2002) Vertical niche differentiation of ectomycorrhizal hyphae in soil as shown by T-RFLP analysis. New Phytol 156:527–535
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J (2014) An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6
Glassman SI, Levine CR, DiRocco AM, Battles JJ, Bruns TD (2016) Ectomycorrhizal fungal spore bank recovery after a severe forest fire: some like it hot. ISME J 10:1228
Glassman SI et al (2015) A continental view of pine-associated ectomycorrhizal fungal spore banks: a quiescent functional guild with a strong biogeographic pattern. New Phytol 205:1619–1631
Guo MS, Ding GD, Gao GL, Zhang Y, Cao HY, Ren Y (2020) Community composition of ectomycorrhizal fungi associated with Pinus sylvestris var. mongolica plantations of various ages in the Horqin Sandy Land. Ecol Indic 110:105860
Gupta P, Vakhlu J, Sharma YP, Imchen M, Kumavath R (2020) Metagenomic insights into the fungal assemblages of the northwest Himalayan cold desert. Extremophiles 24:749–758
Hayward J, Horton TR, Pauchard A, Nunez MA (2015) A single ectomycorrhizal fungal species can enable a Pinus invasion. Ecology 96:1438–1444
Hrynkiewicz K, Baum C, Niedojadło J, Dahm H (2009) Promotion of mycorrhiza formation and growth of willows by the bacterial strain Sphingomonas sp. 23L on fly ash. Biol Fertil Soils 45:385–394
Johnson CN (1996) Interactions between mammals and ectomycorrhizal fungi. Trends Ecol Evol 11:503–507
Kennedy PG, Higgins LM, Rogers RH, Weber MG (2011) Colonization-competition tradeoffs as a mechanism driving successional dynamics in ectomycorrhizal fungal communities. PLoS ONE 6:e25126
Klein T, Hartmann H (2018) Climate change drives tree mortality. Science 362:758–758
Levin AS (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Lilleskov EA, Bruns TD (2005) Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia 97:762–769
Livne-Luzon S, Avidan Y, Weber G, Migael H, Bruns T, Ovadia O, Shemesh H (2016) Wild boars as spore dispersal agents of ectomycorrhizal fungi: consequences for community composition at different habitat types. Mycorrhiza 27:165–174
Livne-Luzon S et al (2017) Small-scale spatial variability in the distribution of ectomycorrhizal fungi affects plant performance and fungal diversity. Ecol Lett 20:1192–1202
Moreira EF, Boscolo D, Viana BF (2015) Spatial heterogeneity regulates plant-pollinator networks across multiple landscape scales. PLoS ONE 10:e0123628
Ne’eman G (1997) Regeneration of natural pine forest–review of work done after the 1989 fire in Mount Carmel, Israel. Int J Wildland Fire 7:295–306
Ne’eman G, Goubitz S, Nathan R (2004) Reproductive traits of Pinus halepensis in the light of fire - a critical review. Plant Ecol 171:69–79
Nguyen NH, Hynson NA, Bruns TD (2012) Stayin’ alive: survival of mycorrhizal fungal propagules from 6-yr-old forest soil. Fungal Ecol 5:741–746. https://doi.org/10.1016/j.funeco.2012.05.006
Nguyen NH et al (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Peay KG (2018) Timing of mutualist arrival has a greater effect on Pinus muricata seedling growth than interspecific competition. J Ecol 106:514–523
Peay KG, Kennedy PG, Bruns TD (2011) Rethinking ectomycorrhizal succession: are root density and hyphal exploration types drivers of spatial and temporal zonation? Fungal Ecol 4:233–240
Pickles BJ, Genney DR, Anderson IC, Alexander IJ (2012) Spatial analysis of ectomycorrhizal fungi reveals that root tip communities are structured by competitive interactions. Mol Ecol 21:5110–5123
Rayner A, Boddy L (1988) Fungal communities in the decay of wood. Adv Microbial Ecol 10:115–166
Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press
Shemesh H, Boaz BE, Millar CI, Bruns TD (2020) Symbiotic interactions above treeline of long-lived pines: Mycorrhizal advantage of limber pine (Pinus flexilis) over Great Basin bristlecone pine (Pinus longaeva) at the seedling stage. J Ecol 108:908–916
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd Edition. Mycorrhizal Symbiosis, 3rd Edition.
Taylor DL, Walters WA, Lennon NJ, Bochicchio J, Krohn A, Caporaso JG, Pennanen T (2016) Accurate estimation of fungal diversity and abundance through improved lineage-specific primers optimized for Illumina Amplicon Sequencing. Appl Environ Microbiol 82:7217–7226
Team RC (2000) R language definition Vienna. R foundation for statistical computing, Austria
Team RC (2018) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Verbruggen E, El Mouden C, Jansa J, Akkermans G, Bücking H, West SA, Kiers ET (2012) Spatial structure and interspecific cooperation: theory and an empirical test using the mycorrhizal mutualism. Am Nat 179:133–146
Yoshida N, Son JA, Matsushita N, Iwamoto K, Hogetsu T (2014) Fine-scale distribution of ectomycorrhizal fungi colonizing Tsuga diversifolia seedlings growing on rocks in a subalpine Abies veitchii forest. Mycorrhiza 24:247–257
Acknowledgements
The authors wish to thank Ofer Ovadia for the ability to use his lab facilities and for his guidance and mentoring, Maren Hale and Liliam Montoya for their technical assistance, Rachel Adams and Cheng Gao for their help with data analysis and Yvonne Lipman for the English editing.
Funding
This research was co-supported by the United States-Israel Binational Science Foundation (BSF Grant 2012081) and Tel-Hai College.
Author information
Authors and Affiliations
Contributions
HS, SLL, OP and TB conceived and designed the experiment. GS and YA performed the experiment. SLL and HS wrote the paper and analyzed the data, and all authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest and that this material has not been submitted for publication elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Livne-Luzon, S., Perlson, O., Avidan, Y. et al. A non-linear effect of the spatial structure of the soil ectomycorrhizal spore bank on the performance of pine seedlings. Mycorrhiza 31, 325–333 (2021). https://doi.org/10.1007/s00572-021-01023-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-021-01023-8