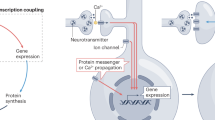
Abstract
The review presents the mechanisms of participation of ions (Na+, K+, and Ca2+) in the processes of synaptic plasticity in the postsynaptic neuron during long-term potentiation and long-term depression. It is assumed that the main participants are AMPA and NMDA receptors, voltage-dependent Na+, K+, Ca2+ channels, Ca2+ and Na+-activated K+ channels, ATP-sensitive K+ channels, and Ca2+ channels of the endoplasmic reticulum. The review provides their molecular characteristics and discusses their role in long-term potentiation and long-term depression. The significance of changes in the intracellular ratio [Na+]i/[K+]i and Ca2+-dependent mechanism are considered for the first time from the signal formation to the level of gene expression. We believe that additional research is needed to identify a subset of neuronal genes whose differential expression contributes to synaptic plasticity, which is implemented with the participation of [Na+]i/[K+]i-sensitive Ca2+-independent “excitation–transcription coupling” mechanism.






Similar content being viewed by others
REFERENCES
Bailey C.H., Kandel E.R., Harris K.M. 2015. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb. Perspect. Biol. 7 (7), 1–29.
Kojima M., Klein R.L., Hatanaka H. 2002. Pre- and post-synaptic modification by neurotrophins. Neurosci. Res. 43 (3), 193–199.
Ghanbari A., Malyshev A., Volgushev M., Stevenson I.H. 2017. Estimating short-term synaptic plasticity from pre- and postsynaptic spiking. PLoS Comput. Biol. 13 (9), 1–28.
Castro-Alamancos M.A., Connors B.W. 1997. Distinct forms of short-term plasticity at excitatory synapses of hippocampus and neocortex. Proc. Natl. Acad. Sci. USA. 94 (8), 4161–4166.
Tallent M.K., Varghis N., Skorobogatko Y., Hernandez-Cuebas L., Whelan K., Vocadlo D.J., Vosseller K. 2009. In vivo modulation of O-GIcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J. Biol. Chem. 284 (1), 174–181.
Hafner A.-S., Donlin-Asp P.G., Leitch B., Herzog E., Schuman E.M. 2019. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science. 364 (6441), eaau3644.
Kukushkin N.V., Carew T.J. 2017. Memory takes time. Neuron. 95 (2), 259–279.
Bliss T.V.P., Collingridge G.L., Morris R.G.M., Reymann K.G. 2018. Long-term potentiation in the hippocampus: Discovery, mechanisms and function. Neuroforum. 24 (3), A103–A120.
Artola A., Singer W. 1993. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 16 (11), 480–487.
Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. 1983. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 305 (5936), 719–721.
Eccles J.C. 1983. Calcium in long-term potentiation as a model for memory. Neuroscience. 10 (4), 1071–1081.
Malenka R.C. 1991. The role of postsynaptic calcium in the induction of long-term potentiation. Mol. Neurobiol. 5 (2–4), 289–295.
Lüscher C., Malenka R.C. 2012. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 4 (6), a005710–a005710.
Flavell S.W., Greenberg M.E. 2008. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 31 (1), 563–590.
Tatsuo S. 1994. Protein kinases involved in the expression of long-term potentiation. Int. J. Biochem. 26 (6), 735–744.
Ma H., Groth R.D., Wheeler D.G., Barrett C.F., Tsien R.W. 2011. Excitation–transcription coupling in sympathetic neurons and the molecular mechanism of its initiation. Neurosci. Res. 70 (1), 2–8.
Orlov S.N., Mongin A.A. 2007. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am. J. Physiol. Circ. Physiol. 293 (4), H2039–H2053.
Sobolevsky A.I., Rosconi M.P., Gouaux E. 2009. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 462 (7274), 745–756.
Linden D.J., Smeyne M., Connor J.A. 1993. Induction of cerebellar long-term depression in culture requires postsynaptic action of Sodium Ions. Neuron. 11 (6), 1093–1100.
Dickenson A.H. 2006. Amino acids: Excitatory. In: Neurotransmitters, Drugs Brain Funct. Chichester, UK: John Wiley & Sons, Ltd, p. 211–223.
Henley J.M., Wilkinson K.A. 2016. Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 17 (6), 337–350.
Luscher C., Malenka R.C. 2012. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 4, a005710.
Lalanne T., Oyrer J., Farrant M., Sjöström P.J. 2018. Synapse type-dependent expression of calcium-permeable AMPA receptors. Front. Synaptic Neurosci. 10, 1–8.
Malinow R., Malenka R.C. 2002. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25 (1), 103–126.
Liu S.Q.J., Cull-Candy S.G. 2000. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 405 (6785), 454–458.
Gielen M., Siegler Retchless B., Mony L., Johnson J.W., Paoletti P. 2009. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 459 (7247), 703–707.
Yuan H., Hansen K.B., Vance K.M., Ogden K.K., Traynelis S.F. 2009. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J. Neurosci. 29 (39), 12045–12058.
Hansen K.B., Furukawa H., Traynelis S.F. 2010. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol. Pharmacol. 78 (4), 535–549.
Karakas E., Furukawa H. 2014. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 344 (6187), 992–997.
Uteshev V. V. 2012. α7 nicotinic ACh receptors as a ligand-gated source of Ca2+ ions: The search for a Ca2+ optimum. Adv Exp Med Biol. 740, 603–638.
Bean B.P. 2007. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8 (6), 451–465.
Yu F.H., Catterall W.A. 2003. Overview of the voltage-gated sodium channel family. Genome Biol. 4 (3), 207.
Catterall W.A. 2000. From ionic currents to molecular mechanisms. Neuron. 26 (1), 13–25.
George A.L. 2005. Inherited disorders of voltage-gated sodium channels. J. Clin. Invest. 115 (8), 1990–1999.
Savio-Galimberti E., Gollob M.H., Darbar D. 2012. Voltage-gated sodium channels: Biophysics, pharmacology, and related channelopathies. Front. Pharmacol. 3, 1–19.
Caldwell J.H., Schaller K.L., Lasher R.S., Peles E., Levinson S.R. 2000. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. USA. 97 (10), 5616–5620.
Hu W., Tian C., Li T., Yang M., Hou H., Shu Y. 2009. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat. Neurosci. 12 (8), 996–1002.
Duflocq A., Le Bras B., Bullier E., Couraud F., Davenne M. 2008. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol. Cell. Neurosci. 39 (2), 180–192.
Solé L., Tamkun M.M. 2020. Trafficking mechanisms underlying Nav channel subcellular localization in neurons. Channels. 14 (1), 1–17.
Kim Y., Hsu C.L., Cembrowski M.S., Mensh B.D., Spruston N. 2015. Dendritic sodium spikes are required for long-term potentiation at distal synapses on hippocampal pyramidal neurons. Elife. 4, 1–30.
Miller C. 2000. An overview of the potassium channel family. Genome Biol. 1 (4), 1–5.
Zemel B.M., Ritter D.M., Covarrubias M., Muqeem T. 2018. A-Type KV channels in dorsal root ganglion neurons: Diversity, function, and dysfunction. Front. Mol. Neurosci. 11, 1–17.
Giese K.P., Storm J.F., Reuter D., Fedorov N.B., Shao L.R., Leicher T., Pongs O., Silva A.J. 1998. Reduced K+ channel inactivation, spike broadening, and after- hyperpolarization in Kvβ1.1-deficient mice with impaired learning. Learn. Mem. 5 (4–5), 257–273.
Hofmann F., Lacinová L., Klugbauer N. 1999. Voltage-dependent calcium channels: From structure to function. Rev. Physiol. Biochem. Pharmacol. 139, 33–87.
Brehm P., Eckert R. 1978. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 202 (4373), 1203–1206.
Striessnig J., Pinggera A., Kaur G., Bock G., Tuluc P. 2014. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 3 (2), 15–38.
Ma H., Cohen S., Li B., Tsien R.W. 2013. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci. Rep. 33 (1), 97–101.
Di Biase V., Obermair G.J., Szabo Z., Altier C., Sanguesa J., Bourinet E., Flucher B.E. 2008. Stable membrane expression of postsynaptic CaV1.2 calcium channel clusters is independent of interactions with AKAP79/150 and APZ proteins. J. Neurosci. 28 (51), 13845–13855.
Jenkins M.A., Christel C.J., Jiao Y., Abiria S., Kim K.Y., Usachev Y.M., Obermair G.J., Colbran R.J., Lee A. 2010. Ca2+-Dependent facilitation of cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 30 (15), 5125–5135.
Striessnig J., Koschak A. 2008. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene Knockout models. Channels. 2 (4), 233–251.
Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K.A., Storm D.R., Hofmann F., Kleppisch T. 2005. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 25 (43), 9883–9892.
White J.A., McKinney B.C., John M.C., Powers P.A., Kamp T.J., Murphy G.G. 2008. Conditional forebrain deletion of the L-type calcium channel Ca V1.2 disrupts remote spatial memories in mice. Learn. Mem. 15 (1), 1–5.
Barad M. 2006. Divide and conquer: An L-type voltage-gated calcium channel subtype finds a role in conditioned fear: Commentary. Learn. Mem. 13 (5), 560–561.
Horrigan F.T., Aldrich R.W. 2002. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120 (3), 267–305.
Rothberg B.S., Magleby K.L. 1999. Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism. J. Gen. Physiol. 114 (1), 93–124.
Berkefeld H., Sailer C.A., Bildl W., Rohde V., Thumfart J.O., Eble S., Klugbauer N., Reisinger E., Bischofberger J., Oliver D., Knaus H.G., Schultes U., Fakler B. 2006. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 314 (5799), 615–620.
Berkefeld H., Fakler B. 2008. Repolarizing responses of BKCa-cav complexes are distinctly shaped by their cav subunits. J. Neurosci. 28 (33), 8238–8245.
Raffaelli G., Saviane C., Mohajerani M.H., Pedarzani P., Cherubini E. 2004. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J. Physiol. 557 (1), 147–157.
Zhang J., Guan X., Li Q., Meredith A.L., Pan H.L., Yan J. 2018. Glutamate-activated BK channel complexes formed with NMDA receptors. Proc. Natl. Acad. Sci. USA. 115 (38), E9006–E9014.
Li Q., Yan J. 2016. Modulation of BK channel function by auxiliary beta and gamma subunits. Physiol. Behav. 128, 51–90.
Li B., Gao T.M. 2016. Functional role of mitochondrial and nuclear BK channels. Int. Rev. Neurobiol. 128, 163–191.
Springer S.J., Burkett B.J., Schrader L.A. 2015. Modulation of BK channels contributes to activity-dependent increase of excitability through MTORC1 activity in CA1 pyramidal cells of mouse hippocampus. Front. Cell. Neurosci. 8, 1–12.
Xia X.M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J.E., Ishii T., Hirschberg B., Bond C.T., Lutsenko S., Maylie J., Adelman J.P. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 395 (6701), 503–507.
Kohler M., Hirschberg B., Bond C.T., Kinzie J.M., Marrion N. V., Maylie J., Adelman J.P. 1996. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 273 (5282), 1709–1714.
Hirschberg B., Maylie J., Adelman J.P., Marrion N. V. 1998. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J. Gen. Physiol. 111 (4), 565–581.
Logsdon N.J., Kang J., Togo J.A., Christian E.P., Aiyar J. 1997. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 272 (52), 32 723–32 726.
Ishii T.M., Silvia C., Hirschberg B., Bond C.T., Adelman J.P., Maylie J. 1997. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA. 94 (21), 11 651–11 656.
Sforna L., Megaro A., Pessia M., Franciolini F., Catacuzzeno L. 2018. Structure, gating and basic functions of the Ca2+-activated K channel of intermediate conductance. Curr. Neuropharmacol. 16 (5), 608–617.
King B., Rizwan A.P., Asmara H., Heath N.C., Engbers J.D.T., Dykstra S., Bartoletti T.M., Hameed S., Zamponi G.W., Turner R.W. 2015. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep. 11 (2), 175–182.
Faber E.S.L., Delaney A.J., Sah P. 2005. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 8 (5), 635–641.
Marrion N. V., Tavalin S.J. 1998. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 395 (6705), 900–905.
Ngo-Anh T.J., Bloodgood B.L., Lin M., Sabatini B.L., Maylie J., Adelman J.P. 2005. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 8 (5), 642–649.
Hammond B., Lemen J., Dudek R., Ward D., Jiang C., Nemeth M., Burns J. 2006. Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem. Toxicol. 44 (2), 147–160.
Dryer S.E. 1994. Na+-activated K+ channels: A new family of large-conductance ion channels. Trends Neurosci. 17 (4), 155–160.
Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. 1984. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 309 (5966), 354–356.
Budelli G., Hage T.A., Wei A., Rojas P., Ivy Jong Y.J., O’Malley K., Salkoff L. 2009. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat. Neurosci. 12 (6), 745–750.
Lu S., Das P., Fadool D.A., Kaczmarek L.K. 2010. The slack sodium-activated potassium channel provides a major outward current in olfactory neurons of Kv1.3–/– super-smeller mice. J. Neurophysiol. 103 (6), 3311–3319.
Nuwer M.O., Picchione K.E., Bhattacharjee A. 2010. PKA-induced internalization of Slack KNa channels produces dorsal root ganglion neuron hyperexcitability. J. Neurosci. 30 (42), 14165–14172.
Hage T.A., Salkoff L. 2012. Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J. Neurosci. 32 (8), 2714–2721.
Kaczmarek L.K. 2013. Slack, slick, and sodium-activated potassium channels. ISRN Neurosci. 2013, 1–14.
Pfeiffer B.E., Huber K.M. 2009. The state of synapses in fragile X syndrome. Neurosci. 15 (5), 549–567.
Noma A. 1983. ATP-regulated K+ channels in cardiac muscle. Nature. 305 (5930), 147–148.
Seino S., Miki T. 2003. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 81 (2), 133–176.
Ashcroft F.M., Harrison D.E., Ashcroft S.J.H. 1984. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature. 312 (5993), 446–448.
Haller M., Mironov S.L., Karschin A., Richter D.W. 2001. Dynamic activation of K ATP channels in rhythmically active neurons. J. Physiol. 537 (1), 69–81.
Zawar C., Plant T.D., Schirra C., Konnerth A., Neumcke B. 1999. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J. Physiol. 514 (2), 327–341.
Choeiri C., Staines W.A., Miki T., Seino S., Renaud J.M., Teutenberg K., Messier C. 2006. Cerebral glucose transporters expression and spatial learning in the K-ATP Kir6.2–/– knockout mice. Behav. Brain Res. 172 (2), 233–239.
Moriguchi S., Ishizuka T., Yabuki Y., Shioda N., Sasaki Y., Tagashira H., Yawo H., Yeh J.Z., Sakagami H., Narahashi T., Fukunaga K. 2018. Blockade of the K ATP channel Kir6.2 by memantine represents a novel mechanism relevant to Alzheimer’s disease therapy. Mol. Psychiatry. 23 (2), 211–221.
Brini M., Carafoli E. 2011. The Plasma Membrane Ca2+ ATPase and the plasma membrane Sodium Calcium exchanger cooperate in the regulation of cell Calcium. Cold Spring Harb. Perspect. Biol. 3 (2), 1–15.
Jaffe D.B., Johnston D., Lasser-Ross N., Lisman J.E., Miyakawa H., Ross W.N. 1992. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 357 (6375), 244–246.
Bennay M., Langer J., Meier S.D., Kafitz K.W., Rose C.R. 2008. Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia. 56 (10), 1138–1149.
Verkhratsky A. 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 85 (1), 201–279.
Block B.A., Imagawa T., Campbell K.P., Franzini-Armstrong C. 1988. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 107 (6), 2587–2600.
Samsó M., Feng W., Pessah I.N., Allen P.D. 2009. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 7 (4), e1000085.
Gao L., Tripathy A., Lu X., Meissner G. 1997. Evidence for a role of C-terminal amino acid residues in skeletal muscle Ca2+ release channel (ryanodine receptor) function. FEBS Lett. 412 (1), 223–226.
Kunerth S., Langhorst M.F., Schwarzmann N., Gu X., Huang L., Yang Z., Zhang L., Mills S.J., Zhang L.H., Potter B.V.L., Guse A.H. 2004. Amplification and propagation of pacemaker Ca2+ signals by cyclic ADP-ribose and the type 3 ryanodine receptor in T cells. J. Cell Sci. 117 (10), 2141–2149.
Gerasimenko J. V., Flowerdew S.E., Voronina S.G., Sukhomlin T.K., Tepikin A. V., Petersen O.H., Gerasimenko O. V. 2006. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J. Biol. Chem. 281 (52), 40154–40163.
Sukhareva M., Smith S. V., Maric D., Barker J.L. 2002. Functional properties of ryanodine receptors in hippocampal neurons change during early differentiation in culture. J. Neurophysiol. 88 (3), 1077–1087.
Korkotian E., Segal M. 2011. Synaptopodin regulates release of calcium from stores in dendritic spines of cultured hippocampal neurons. J. Physiol. 589 (24), 5987–5995.
Raymond C.R., Redman S.J. 2002. Different calcium sources are narrowly tuned to the induction of different forms of LTP. J. Neurophysiol. 88 (1), 249–255.
Raymond C.R., Redman S.J. 2006. Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J. Physiol. 570 (1), 97–111.
Balschun D., Wolfer D.P., Bertocchini F., Barone V., Conti A., Zuschratter W., Missiaen L., Lipp H.P., Frey J.U., Sorrentino V. 1999. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J. 18 (19), 5264–5273.
Santulli G., Nakashima R., Yuan Q., Marks A.R. 2017. Intracellular calcium release channels: An uAPate. J. Physiol. 595 (10), 3041–3051.
Bezprozvanny I., Ehrlich B.E. 1994. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: Conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 104 (5), 821–856.
Boehning D., Mak D.O.D., Foskett J.K., Joseph S.K. 2001. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J. Biol. Chem. 276 (17), 13 509–13 512.
Boehning D., Joseph S.K., Mak D.O.D., Foskett J.K. 2001. Single-channel recordings of recombinant inositol trisphosphate receptors in mammalian nuclear envelope. Biophys. J. 81 (1), 117–124.
Marchenko S.M., Yarotskyy V. V., Kovalenko T.N., Kostyuk P.G., Thomas R.C. 2005. Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones. J. Physiol. 565 (3), 897–910.
Fujii S., Matsumoto M., Igarashi K., Kato H., Mikoshiba K. 2000. Synaptic plasticity in hippocampal CA1 neurons of mice lacking type 1 inositol-1,4,5-trisphosphate receptors. Learn. Mem. 7 (5), 312–320.
Qiu Z., Nicoll D.A., Philipson K.D. 2001. Helix packing of functionally important regions of the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 276 (1), 194–199.
Nicoll D.A., Ottolia M., Lu L., Lu Y., Philipson K.D. 1999. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 274 (2), 910–917.
Hilge M., Aelen J., Vuister G.W. 2006. Ca2+ Regulation in the Na+ /Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol. Cell. 22 (1), 15–25.
Lytton J. 2007. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 406 (3), 365–382.
Quednau B.D., Nicoll D.A., Philipson K.D. 2004. The sodium/calcium exchanger family SLC8. Pflügers Arch. Eur. J. Physiol. 447 (5), 543–548.
Quednau B.D., Nicoll D.A., Philipson K.D. 1997. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. Physiol. 272 (4), C1250–C1261.
DiPolo R., Beaugé L. 2006. Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiol. Rev. 86 (1), 155–203.
Blaustein M.P., Juhaszova M., Golovina V.A., Church P.J., Stanley E.F. 2006. Na/Ca exchanger and PMCA localization in neurons and astrocytes. Ann. N. Y. Acad. Sci. 976 (1), 356–366.
Jeon D., Yang Y.M., Jeong M.J., Philipson K.D., Rhim H., Shin H.S. 2003. Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron. 38 (6), 965–976.
Molinaro P., Viggiano D., Nisticò R., Sirabella R., Secondo A., Boscia F., Pannaccione A., Scorziello A., Mehdawy B., Sokolow S., Herchuelz A., di Renzo G.F., Annunziato L. 2011. Na+-Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long-term potentiation and spatial learning and memory. J. Neurosci. 31 (20), 7312–7321.
Scheiner-Bobis G. 2002. The sodium pump. Eur. J. Biochem. 269 (10), 2424–2433.
Blanco G. 2005. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 25 (5), 292–303.
Hieber V., Siegel G.J., Fink D.J., Beaty M.W., Mata M. 1991. Differential distribution of (Na, K)-ATPase? isoforms in the central nervous system. Cell. Mol. Neurobiol. 11 (2), 253–262.
McGrail K., Phillips J., Sweadner K. 1991. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: Both neurons and glia can express more than one Na,K-ATPase. J. Neurosci. 11 (2), 381–391.
Peng L., Martin-Vasallo P., Sweadner K.J. 1997. Isoforms of Na,K-ATPase α and β subunits in the rat cerebellum and in granule cell cultures. J. Neurosci. 17 (10), 3488–3502.
Akkuratov E.E., Lopacheva O.M., Kruusmägi M., Lopachev A. V., Shah Z.A., Boldyrev A.A., Liu L. 2015. Functional interaction between Na/K-ATPase and NMDA receptor in cerebellar neurons. Mol. Neurobiol. 52 (3), 1726–1734.
Munzer J.S., Daly S.E., Jewell-Motz E.A., Lingrel J.B., Blostein R. 1994. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J. Biol. Chem. 269 (24), 16 668–16 676.
Jewell E.A., Lingrel J.B. 1991. Comparison of the substrate dependence properties of the rat Na,K-ATPase α1, α2, and α3 isoforms expressed in HeLa cells. J. Biol. Chem. 266 (25), 16 925–16 930.
Zahler R., Zhang Z.-T., Manor M., Boron W.F. 1997. Sodium kinetics of Na,K-ATPase α isoforms in intact transfected HeLa cells. J. Gen. Physiol. 110 (2), 201–213.
Dobretsov M. 2005. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front. Biosci. 10, 2373–2396.
Azarias G., Kruusmägi M., Connor S., Akkuratov E.E., Liu X.-L., Lyons D., Brismar H., Broberger C., Aperia A. 2013. A Specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3. J. Biol. Chem. 288 (4), 2734–2743.
Kim J.H., Sizov I., Dobretsov M., Von Gersdorff H. 2007. Presynaptic Ca2+ buffers control the strength of a fast post-tetanic hyperpolarization mediated by the α3 Na+/K+-ATPase. Nat. Neurosci. 10 (2), 196–205.
Glushchenko T.S., Izvarina N.L. 1997. Na+,K+-ATPase activity in neurons and glial cells of the olfactory cortex of the rat brain during the development of long-term potentiation. Neurosci. Behav. Physiol. 27 (1), 49–52.
Reich C.G., Mason S.E., Alger B.E. 2004. Novel form of LTD induced by transient, partial inhibition of the Na, K-pump in rat hippocampal CA1 cells. J. Neurophysiol. 91 (1), 239–247.
Niggli V., Sigel E., Carafoli E. 1982. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J. Biol. Chem. 257 (5), 2350–2356.
Smallwood J.I., Waisman D.M., Lafreniere D., Rasmussen H. 1983. Evidence that the erythrocyte calcium pump catalyzes a Ca2+:nH+ exchange. J. Biol. Chem. 258 (18), 11 092–11 097.
Di Leva F., Domi T., Fedrizzi L., Lim D., Carafoli E. 2008. The plasma membrane Ca2+ ATPase of animal cells: Structure, function and regulation. Arch. Biochem. Biophys. 476 (1), 65–74.
Greeb J., Shull G.E. 1989. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J. Biol. Chem. 264 (31), 18 569–18 576.
Stauffer T.P., Guerini D., Carafoli E. 1995. Tissue distribution of the four gene products of the plasma membrane Ca pump. J. Biol. Chem. 270 (20), 12184–12190.
Mata A.M. 2010. Plasma membrane Ca2+-ATPases in the nervous system during development and ageing. World J. Biol. Chem. 1 (7), 229.
Burette A.C., Strehler E.E., Weinberg R.J. 2009. ‘Fast’ plasma membrane calcium pump PMCA2a concentrates in GABAergic terminals in the adult rat brain. J. Comp. Neurol. 512 (4), 500–513.
Strehler E.E., Filoteo A.G., Penniston J.T., Caride A.J. 2007. Plasma-membrane Ca2+ pumps: Structural diversity as the basis for functional versatility. Biochem. Soc. Trans. 35 (5), 919–922.
Marques-da-Silva D., Gutierrez-Merino C. 2014. Caveolin-rich lipid rafts of the plasma membrane of mature cerebellar granule neurons are microcompartments for calcium/reactive oxygen and nitrogen species cross-talk signaling. Cell Calcium. 56 (2), 108–123.
Garside M.L., Turner P.R., Austen B., Strehler E.E., Beesley P.W., Empson R.M. 2009. Molecular interactions of the plasma membrane calcium ATPase 2 at pre- and post-synaptic sites in rat cerebellum. Neuroscience. 162 (2), 383–395.
Zanni G., Calì T., Kalscheuer V.M., Ottolini D., Barresi S., Lebrun N., Montecchi-Palazzi L., Hu H., Chelly J., Bertini E., Brini M., Carafoli E. 2012. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc. Natl. Acad. Sci. USA. 109 (36), 14 514–14 519.
Kip S.N., Gray N.W., Burette A., Canbay A., Weinberg R.J., Strehler E.E. 2006. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus. 16 (1), 20–34.
Misquitta C.M., Mack D.P., Grover A.K. 1999. Sarco/endoplasmic reticulum Ca2+(SERCA)-pumps: Link to heart beats and calcium waves. Cell Calcium. 25 (4), 277–290.
Verboomen H., Wuytack F., Van den Bosch L., Mertens L., Casteels R. 1994. The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca2+-transport ATPase (SERCA2a/b). Biochem. J. 303 (3), 979–984.
Majewska A., Brown E., Ross J., Yuste R. 2000. Mechanisms of calcium decay kinetics in hippocampal spines: Role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J. Neurosci. 20 (5), 1722–1734.
Emptage N.J., Reid C.A., Fine A. 2001. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 29 (1), 197–208.
Collingridge B.Y.G.L., Kehl S.J., Mclennan H. 1983. Excitatory amino acids in synaptic transmission in the schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 334, 33–46.
Bliss T. V., Collingridge G.L. 1993. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 361 (6407), 31–39.
Aniksztejn L., Ben-Ari Y. 1995. Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J. Neurophysiol. 74 (6), 2349–2357.
Berridge M.J. 1998. Neuronal calcium signaling. Neuron. 21 (1), 13–26.
Malenka R.C., Lancaster B., Zucker R.S. 1992. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron. 9 (1), 121–128.
Santana L.F. 2008. Editorial: NFAT-dependent excitation-transcription coupling in heart. Circ. Res. 103 (7), 681–683.
Gundersen K. 2011. Excitation-transcription coupling in skeletal muscle: The molecular pathways of exercise. Biol. Rev. 86 (3), 564–600.
Morgan J.I., Curran T. 1989. Stimulus-transcription coupling in neurons: Role of cellular immediate-early genes. Trends Neurosci. 12 (11), 459–462.
Sheng M., Greenberg M.E. 1990. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 4 (4), 477–485.
Cole A.J., Saffen D.W., Baraban J.M., Worley P.F. 1989. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 340 (6233), 474–476.
Fleischmann A., Hvalby O., Jensen V., Strekalova T., Zacher C., Layer L.E., Kvello A., Reschke M., Spanagel R., Sprengel R., Wagner E.F., Gass P. 2003. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-fos in the CNS. J. Neurosci. 23 (27), 9116–9122.
Minatohara K., Akiyoshi M., Okuno H. 2016. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front. Mol. Neurosci. 8, 1–11.
Greenberg M.E., Ziff E.B., Greene L.A. 1986. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 234 (4772), 80–83.
Orlov S.N., Aksentsev S.L., Kotelevtsev S. V. 2005. Extracellular calcium is required for the maintenance of plasma membrane integrity in nucleated cells. Cell Calcium. 38 (1), 53–57.
Koltsova S. V., Tremblay J., Hamet P., Orlov S.N. 2015. Transcriptomic changes in Ca2+-depleted cells: Role of elevated intracellular [Na+]/[K+] ratio. Cell Calcium. 58 (3), 317–324.
Deisseroth K., Heist E.K., Tsien R.W. 1998. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 392 (6672), 198–202.
Ma H., Groth R.D., Cohen S.M., Emery J.F., Li B., Hoedt E., Zhang G., Neubert T.A., Tsien R.W. 2014. γcaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell. 159 (2), 281–294.
Malenka R.C., Kauer J.A., Perkel D.J., Mauk M.D., Kelly P.T., Nicoll R.A., Waxham M.N. 1989. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 340 (6234), 554–557.
Mcdonald T.F., Pelzer S., Trautwein W., Pelzer D.J. 1994. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 74 (2), 365–507.
Greer P.L., Greenberg M.E. 2008. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron. 59 (6), 846–860.
Clapham D.E. 2007. Calcium signaling. Cell. 131 (6), 1047–1058.
Derkach V., Barria A., Soderling T.R. 1999. Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA. 96 (6), 3269–3274.
Kristensen A.S., Jenkins M. a., Banke T.G., Schousboe A., Johnson R.C., Huganir R., Traynelis S.F. 2011. Mechanism of CaMKII regulation of AMPA receptor gating. Nat. Neurosci. 14 (6), 727–735.
Mitsushima D., Ishihara K., Sano A., Kessels H.W., Takahashi T. 2011. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc. Natl. Acad. Sci. USA. 108 (30), 12 503–12 508.
Banke T.G., Bowie D., Lee H., Huganir R.L., Schousboe A., Traynelis S.F. 2000. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 20 (1), 89–102.
Chetkovich D.M., Gray R., Johnston D., Sweatt J.D. 1991. N-Methyl-D-aspartate receptor activation increases cAMP levels and voltage-gated Ca2+ channel activity in area CA1 of hippocampus. Proc. Natl. Acad. Sci. USA. 88 (15), 6467–6471.
Lau C.G., Takeuchi K., Rodenas-Ruano A., Takayasu Y., Murphy J., Bennett M.V.I., Zukin R.S. 2009. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem. Soc. Trans. 37 (6), 1369–1374.
Aman T.K., Maki B.A., Ruffino T.J., Kasperek E.M., Popescu G.K. 2014. Separate intramolecular targets for protein kinase A control N-Methyl-D-aspartate receptor gating and Ca2+ permeability. J. Biol. Chem. 289 (27), 18 805–18 817.
Matthews R.P., Guthrie C.R., Wailes L.M., Zhao X., Means A.R., McKnight G.S. 1994. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol. Cell. Biol. 14 (9), 6107–6116.
Reymann K.G., Frey U., Jork R., Matthies H. 1988. Polymyxin B, an inhibitor of protein kinase C, prevents the maintenance of synaptic long-term potentiation in hippocampal CA1 neurons. Brain Res. 440 (2), 305–314.
Kauer J.A., Malenka R.C., Nicoll R.A. 1988. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1 (10), 911–917.
Lovinger D.M., Wong K.L., Murakami K., Routtenberg A. 1987. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 436 (1), 177–183.
Finkbeiner S., Greenberg M.E. 1996. Ca2+-dependent routes to Ras: Mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 16 (2), 233–236.
Herring B.E., Nicoll R.A. 2016. Long-term potentiation: From CaMKII to AMPA receptor trafficking. Annu. Rev. Physiol. 78 (1), 351–365.
Murakoshi H., Wang H., Yasuda R. 2011. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 472 (7341), 100–104.
Lynch G., Rex C.S., Gall C.M. 2007. LTP consolidation: Substrates, explanatory power, and functional significance. Neuropharmacology. 52 (1), 12–23.
Amini M., Ma C. -l., Farazifard R., Zhu G., Zhang Y., Vanderluit J., Zoltewicz J.S., Hage F., Savitt J.M., Lagace D.C., Slack R.S., Beique J.-C., Baudry M., Greer P.A., Bergeron R., Park D.S. 2013. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J. Neurosci. 33 (13), 5773–5784.
Baeza-Lehnert F., Saab A.S., Gutiérrez R., Larenas V., Díaz E., Horn M., Vargas M., Hösli L., Stobart J., Hirrlinger J., Weber B., Barros L.F. 2019. Non-canonical control of neuronal energy status by the Na+ pump. Cell Metab. 29 (3), 668–680.e4.
Kiedrowski L., Wroblewski J.T., Costa E. 1994. Intracellular sodium concentration in cultured cerebellar granule cells challenged with glutamate. Mol. Pharmacol. 45 (5), 1050–1054.
Rose C.R., Konnerth A. 2001. NMDA receptor-mediated Na+ signals in spines and dendrites. J. Neurosci. 21 (12), 4207–4214.
Somjen G.G. 2002. Ion regulation in the brain: Implications for pathophysiology. Neuroscientist. 8 (3), 254–267.
Deitmer J.W., Rose C.R. 2010. Ion changes and signalling in perisynaptic glia. Brain Res. Rev. 63 (1–2), 113–129.
Verkhratsky A., Noda M., Parpura V., Kirischuk S. 2013. Sodium fluxes and astroglial function. Adv. Exp. Med. Biol. 961, 295–305.
Callaway J.C., Ross W.N. 1997. Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J. Neurophysiol. 77 (1), 145–152.
Miyazaki K., Ross W.N. 2017. Sodium dynamics in pyramidal neuron dendritic spines: Synaptically evoked entry predominantly through AMPA receptors and removal by diffusion. J. Neurosci. 37 (41), 9964–9976.
Behnisch T., Reymann K.G. 1995. Thapsigargin blocks long-term potentiation induced by weak, but not strong tetanisation in rat hippocampal CA1 neurons. Neurosci. Lett. 192 (3), 185–188.
Balschun D., Wolfer D.P., Gass P., Mantamadiotis T., Welzl H., Schütz G., Frey J.U., Lipp H.P. 2003. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 23 (15), 6304–6314.
Koltsova S. V., Trushina Y., Haloui M., Akimova O.A., Tremblay J., Hamet P., Orlov S.N. 2012. Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: Evidence for Ca2+ i-independent excitation-transcription coupling. PLoS One. 7 (5), e38032.
Kasahara J., Fukunaga K., Miyamoto E. 2001. Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J. Biol. Chem. 276 (26), 24 044–24 050.
Taurin S., Dulin N.O., Pchejetski D., Grygorczyk R., Tremblay J., Hamet P., Orlov S.N. 2002. c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: Evidence for an intracellular-sodium-mediated, calcium-independent mechanism. J. Physiol. 543 (3), 835–847.
Haloui M., Taurin S., Akimova O.A., Guo D.F., Tremblay J., Dulin N.O., Hamet P., Orlov S.N. 2007. [Na+]i-induced c-Fos expression is not mediated by activation of the 5′-promoter containing known transcriptional elements. FEBS J. 274 (14), 3557–3567.
Orlov S.N., Taurin S., Tremblay J., Hamet P. 2001. Inhibition of Na+,K+ pump affects nucleic acid synthesis and smooth muscle cell proliferation via elevation of the [Na+]i/ [K+]i ratio: Possible implication in vascular remodelling. J. Hypertens. 19 (9), 1559–1565.
Smolyaninova L. V., Shiyan A.A., Kapilevich L. V., Lopachev A. V., Fedorova T.N., Klementieva T.S., Moskovtsev A.A., Kubatiev A.A., Orlov S.N. 2019. Transcriptomic changes triggered by ouabain in rat cerebellum granule cells: Role of α3- And α1- Na+,K+-ATPase-mediated signaling. PLoS One. 14 (9), 1–23.
Yang H., Chen C. 2008. Cyclooxygenase-2 in synaptic signaling. Curr. Pharm. Des. 14 (14), 1443–1451.
Yamagata K., Andreasson K.I., Kaufmann W.E., Barnes C.A., Worley P.F. 1993. Expression of a mitogen-inducible cyclooxygenase in brain neurons: Regulation by synaptic activity and glucocorticoids. Neuron. 11 (2), 371–386.
Chen C., Magee J.C., Bazan N.G. 2002. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 87 (6), 2851–2857.
Sang N., Chen C. 2006. Lipid signaling and synaptic plasticity. Neuroscientist. 12 (5), 425–434.
Yagami T., Koma H., Yamamoto Y. 2016. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol. Neurobiol. 53 (7), 4754–4771.
Koelle M.R. 1997. A new family of G-protein regulators – the RGS proteins. Curr. Opin. Cell Biol. 9 (2), 143–147.
Ingi T., Krumins A.M., Chidiac P., Brothers G.M., Chung S., Snow B.E., Barnes C.A., Lanahan A.A., Siderovski D.P., Ross E.M., Gilman A.G., Worley P.F. 1998. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J. Neurosci. 18 (18), 7178–7188.
Leslie J.H., Nedivi E. 2011. Activity-regulated genes as mediators of neural circuit plasticity. Prog. Neurobiol. 94 (3), 223–237.
Rudy J.W. 2014. The neurobiology of learning and memory. Sunderland, MA: Sinauer Associates, Inc. Publishers, p. 48–55.
Xu X., Shrager P. 2005. Dependence of axon initial segment formation on Na+ channel expression. J. Neurosci. Res. 79 (4), 428–441.
Oda T., Makino K., Yamashita I., Namba K., Maéda Y. 2001. Distinct structural changes detected by X-ray fiber diffraction in stabilization of F-actin by lowering pH and increasing ionic strength. Biophys. J. 80 (2), 841–851.
Rajamanickam G.D., Kroetsch T., Kastelic J.P., Thundathil J.C. 2017. Testis-specific isoform of Na/K-ATPase (ATP1A4) regulates sperm function and fertility in dairy bulls through potential mechanisms involving reactive oxygen species, calcium and actin polymerization. Andrology. 5 (4), 814–823.
Rajasekaran A.K., Rajasekaran S.A. 2003. Role of Na-K-ATPase in the assembly of tight junctions. Am. J. Physiol. – Ren. Physiol. 285, 388–396.
La J., Reed E.B., Koltsova S., Akimova O., Hamanaka R.B., Mutlu G.M., Orlov S.N., Dulin N.O. 2016. Regulation of myofibroblast differentiation by cardiac glycosides. Am. J. Physiol. – Lung Cell. Mol. Physiol. 310 (9), L815–L823.
Alder J., Thakker-Varia S., Bangasser D.A., Kuroiwa M., Plummer M.R., Shors T.J., Black I.B. 2003. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J. Neurosci. 23 (34), 10 800–10 808.
Altar C.A., Laeng P., Jurata L.W., Brockman J.A., Lemire A., Bullard J., Bukhman Y. V., Young T.A., Charles V., Palfreyman M.G. 2004. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J. Neurosci. 24 (11), 2667–2677.
Sidorenko S., Klimanova E., Milovanova K., Lopina O.D., Kapilevich L.V., Chibalin A.V., Orlov S.N. 2018. Transcriptomic changes in C2C12 myotubes triggered by electrical stimulation: Role of \({\text{Ca}}_{{\text{i}}}^{{2 + }}\)-mediated and \({\text{Ca}}_{{\text{i}}}^{{2 + }}\)-independent signaling and elevated [Na+]i/[K+]i ratio. Cell Calcium. 76, 72–86.
Ng A.N., Toresson H. 2011. Endoplasmic reticulum dynamics in hippocampal dendritic spines induced by agonists of type I metabotropic glutamate but not by muscarinic acetylcholine receptors. Synapse. 65 (4), 351–355.
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 19-14-50 358).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that there is no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Puchkov
Abbreviations: AMPAR, AMPA receptors; AP, action potential; APV, aminophosphovaleric acid; CaM, calmodulin; CaV, voltage-dependent Ca2+ channels; CIRC, calcium-induced release of calcium; CNQX, cyanquinoxalin; CP-AMPAR, Ca2+-permeable AMPA receptors; EPR, endoplasmic reticulum; ETC, excitation–transcription coupling; IAS, initial axon segments; IEG, immediate early genes; InsP3R, inositol-3-phosphate receptors; LTD, long-term depression; LTP, long-term potentiation; KATP, ATP-sensitive K+ channels; KCa, Ca2+-activated K+ channels; KNa, Na+-activated K+ channels; KV, voltage-dependent K+ channels; MP, membrane potential; NaV, voltage-dependent Na+ channels; NCX, Na+/Ca2+ exchanger; NMDAR, NMDA receptors; PMCA, plasma membrane Ca2+-ATPase; RP, resting potential; RyR, ryanodine receptors; SERCA, endoplasmic reticulum Ca2+-ATPase.
Rights and permissions
About this article
Cite this article
Smolyaninova, L.V., Shiyan, A.A., Maksimov, G.V. et al. Contribution of Monovalent (Na+ and K+) and Divalent (Ca2+) Ions to the Mechanisms of Synaptic Plasticity. Biochem. Moscow Suppl. Ser. A 15, 1–20 (2021). https://doi.org/10.1134/S1990747820050062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747820050062