Abstract
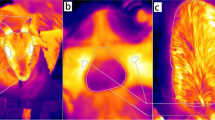
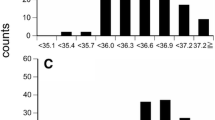
The study aimed at comparing variations in body temperature values recorded using rectal digital, infrared, and mercury-in-glass thermometers in donkeys during the hot-dry season, prevailing under tropical savannah conditions. Thirty donkeys that served as subjects were divided into three groups of adults, yearlings, and foals. Values of the body temperature of each donkey were recorded bihourly, starting from 06:00 h till 18:00 h, by digital (5-cm depth of insertion), mercury-in-glass (3 cm depth), and infrared thermometers. The values obtained by each type of the thermometer were compared with those recorded using a 15-cm digital probe (Model HI935007, Hanna Instruments, range −50.0 to 150.0°C; accuracy ± 0.2°C) which served as the gold standard. Dry-bulb temperature (34.00 ± 0.50°C), temperature-humidity index (79.65 ± 0.15), and wet-bulb globe temperature (28.00 ± 0.50) index peaked at 14:00 h. The mean body temperatures for rectal probe, digital, mercury-in-glass, and infrared thermometers were 38.35 ± 0.11°C, 37.24 ± 0.04°C, 36.76 ± 0.06°C, and 36.92 ± 0.07°C, respectively. In comparison to the rectal probe, the mean bias for digital (−1.11 ± 0.05°C), mercury-in-glass (−1.59 ± 0.07°C), and infrared thermometers (−1.38 ± 0.07°C) was large. The Passing-Bablok regression plot demonstrated significant deviation from linearity (p < 0.01) when digital, infrared, and mercury-in-glass thermometers were compared to the rectal probe. The area under the curve (AUC) for digital (AUC: 0.7005 ± 0.01 [95%: 0.6853 – 0.7310], infrared (AUC: 0.6711 ± 0.01 [95%: 0.6322 – 0.7100], and mercury-in-glass (AUC: 0.6321 ± 0.01 [95%: 0.6001 – 0.7873] thermometers showed poor accuracy with low sensitivity. In conclusion, the use of digital, mercury-in-glass, and infrared thermometers in recording body temperature in donkeys during the hot-dry season underestimated the values. Their use in measuring body temperature may result in wrong diagnosis, and compromise the control of hyperthermia and diseases associated with thermoregulatory impairments in donkeys.








Similar content being viewed by others
References
Adams AE, Olea-Popelka FJ, Roman-Muniz IN (2013) Using temperature-sensing reticular boluses to aid in the detection of production diseases in dairy cows. J Dairy Sci 96:1549–1555
Akobeng AK (2007) Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 96:644–647
Akpa GN, Ifut JO, Mohammed F (2002) Indigenous management of dystocia in ruminant livestock of Northern Guinea Savannah of Nigeria. Niger J Anim Prod 29:264–270
American Psychological Association (2002) Ethical principles of psychologists and code of conduct (2002, Amended June 1, 2010). http://www.apa.org/ethics/code/index.aspx. Accessed on 4 December 2019; 2010.
Ayo JO, Dzenda T, Olaifa F, Ake SA, Sani I (2014) Diurnal and seasonal fluctuations in rectal temperature, respiration and heart rate of pack donkeys in a tropical Savannah zone. J Equine Sci 25:1–6
Bartolomé E, Sánchez MJ, Molina A, Schaefer AL, Cervantes I, Valera M (2013) Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible influence on sport performance. Animal 7(12):2044–2053
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Bland JM, Altman DG (2003) Interaction revisited: the difference between two estimates. BMJ 326(7382):219. https://doi.org/10.1136/bmj.326.7382.219
Bland JM, Altman DG (2010) Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud 47(8):931–936
Burfeind O, Von Keyserlingk MAG, Weary DM, Veira DM, Heuwieser W (2010) Repeatability of measures of rectal temperature in dairy cows. J Dairy Sci 93(2):624–627
Church JS, Hegadoren PR, Paetkau MJ, Miller CC, Regev-Shoshani G, Schaefer AL, Schwartzkopf-Genswein KS (2014) Influence of environmental factors on infrared eye temperature measurements in cattle. Res Vet Sci 96(1):220–226
de Carvalho Ferreira HC, Weesendorp E, Elbers ARW, Bouma A, Quak S, Stegeman JA, Loeffen WLA (2012) African swine fever virus excretion patterns in persistently infected animals: a quantitative approach. Vet Microbiol 160:327–340
de Melo Costa CC, Campos Maia AS, Brown-Brandl TM, Chiquitelli Neto M, de França Carvalho Fonsêca V (2018) Thermal equilibrium of Nellore cattle in tropical conditions: an investigation of circadian pattern. J Therm Biol 74:317–324
d'Eath RB, Turner SP, Kurt E, Evans G, Thölking L, Looft H, Ison SH (2010) Pigs’ aggressive temperament affects pre-slaughter mixing aggression, stress and meat quality. Animal 4:604–616
Duru CO, Akinbami FO, Orimadegun AE (2012) A comparison of tympanic and rectal temperatures in term Nigerian neonates. BMC Pediatr 12:86 http://www.biomedcentral.com/1471-2431/12/86
El-Radhi AS (2018) Measurement of body temperature. In: El-Radhi A (eds) Clinical manual of fever in children. Cham, Switzerland: Springer, pp 69–84. https://doi.org/10.1007/978-3-319-92336-9_4.
Ferreira-Silva JC, Nascimento PS, Azevedo MV, Rocha JM, Ferreira HN, Moura MT, Oliveira MAL (2017) Evaluation of clinical and reproductive parameters in Mangalarga Marchador mares treated with different doses of Cloprostenol or Dinoprost. Med Vet 11(2):137–144
Fielding D, Krause P (1998) The tropical agriculturist series. In: Coste R, Smith AJ (eds) Macmillan Education Ltd. London and Basingstoke, London, p 119
Fulbrook P (1993) Core temperature measurement in adults: a literature review. J Adv Nurs 18(9):1451–1460
Giannetto C, Fazio F, Vazzana I, Panzera M, Piccione G (2012) Comparison of cortisol and rectal temperature circadian rhythms in horses: the role of light/dark cycle and constant darkness. Biol Rhythm Res 43(6):681–687
Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH (2002) Effects of size and temperature on developmental time. Nature 417:70–73
Green AR, Gates RS, Lawrence LM (2005) Measurement of horse core body temperature. J Therm Biol 30(5):370–377
Hall EJ, Carter AJ, Stevenson AG, Hall C (2019) Establishing a yard-specific normal rectal temperature reference range for horses. J Equine Vet Sci 74:51–55
Hill TM, Bateman HG II, Suarez-Mena FX, Dennis TS, Schlotterbeck RL (2016) Changes in body temperature of calves up to 2 months of age as affected by time of day, age, and ambient temperature. J Dairy Sci 99(11):8867–8870
Hine L, Laven RA, Sahu SK (2015) An analysis of the effect of thermometer type and make on rectal temperature measurements of cattle, horses and sheep. N Z Vet J 63(3):171–173
Hines MT (2004) Changes in body temperature, Eds. In: Reed SM, Bayly WM (eds) Equine Internal Medicine. Elsevier, St. Louis, pp 148–155
Holcomb KE (2017) Is shade for horses a comfort resource or a minimum requirement. J Anim Sci 95(9):4206–4212
Holcomb KE, Tucker CB, Stull CL (2013) Physiological, behavioral, and serological responses of horses to shaded or unshaded pens in a hot, sunny environment. J Anim Sci 91(12):5926–5936
Jansson A, Lindgren G, Velie BD, Solé M (2020) An investigation into factors influencing basal eye temperature in the domestic horse (Equus caballus) when measured using infrared thermography in field conditions. Physiol Behav 228:113218
Johnson SR, Rao S, Hussey SB, Morley PS, Traub-Dargatz JL (2011) Thermographic eye temperature as an index to body temperature in ponies. J Equine Vet Sci 31:63–66
Khaliq I, Hof C, Prinzinger R, Boehning-Gaese K, Pfenninger M (2014) Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc R Soc B Biol Sci 281(1789):20141097. https://doi.org/10.1098/rspb.2014.1097
Ko Y, Jung JY, Kim HT, Lee JY (2019) Auditory canal temperature measurement using a wearable device during sleep: comparisons with rectal temperatures at 6, 10, and 14 cm depths. J Therm Biol 85:102410. https://doi.org/10.1016/j.jtherbio.2019.102410
Kumar R, Indrayan A (2011) Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr 48:277–287. https://doi.org/10.1007/s13312-011-0055-4
Lee JY, Wakabayashi H, Wijayanto T, Tochihara Y (2010) Differences in rectal temperatures measured at depths of 4–19 cm from the anal sphincter during exercise and rest. Eur J Appl Physiol 109(1):73–80
Lichtenberger M, Lennox AM (2012) Critical care of the exotic companion mammal (with a focus on herbivorous species): the first twenty-four hours. J Exot Pet Med 21:284–292
Lima SB, Stafuzza NB, Pires BV, Bonilha SF, Cyrillo JN, Negrão JA, Paz CC (2020) Effect of high temperature on physiological parameters of Nelore (Bos taurus indicus) and Caracu (Bos taurus taurus) cattle breeds. Trop Anim Health Prod 52:1–9. https://doi.org/10.1007/s11250-020-02249-y
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2007) Physiological traits as affected by heat stress in sheep - a review. Small Rumin Res 71:1–12
McCafferty DJ, Gallon S, Nord A (2015) Challenges of measuring body temperatures of free-ranging birds and mammals. Anim Biotelemetry 3(1):33. https://doi.org/10.1186/s40317-015-0075-2
McCallum L, Higgins D (2012) Measuring body temperature. Nurs Times 108:20–22
McGreevy P, Warren-Smith A, Guisard Y (2012) The effect of double bridles and jaw-clamping crank nosebands on temperature of eyes and facial skin of horses. J Vet Behav 7:142–148
McKeever KH, Eaton TL, Geiser S, Kearns CF, Lehnhard RA (2010) Age related decreases in thermoregulation and cardiovascular function in horses: aging and thermoregulation. Equine Vet J 42:220–227
McManus C, Louvandini H, Gugel R, Sasaki LCB, Bianchini E, Bernal FEM, Paim TP (2011) Skin and coat traits in sheep in Brazil and their relation with heat tolerance. Trop Anim Health Prod 43(1):121–126
McNicholl J, Howarth GS, Hazel SJ (2016) Influence of the environment on body temperature of racing greyhounds. Front Vet Sci 3:53. https://doi.org/10.3389/fvets.2016.00053
Naylor JM, Streeter RM, Torgerson P (2012) Factors affecting rectal temperature measurement using commonly available digital thermometers. Res Vet Sci 92(1):121–123
Passing H, Bablok W (1983) A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. J Clin Chem Clin Biochem 21:709–720
Passing H, Bablok W (1984) Comparison of several regression procedures for method comparison studies and determination of sample sizes application of linear regression procedures for method comparison studies in clinical chemistry, part II. J Clin Chem Clin Biochem 22:431–445
Pearson RA, Ouassar M (2000) A Guide to live weight estimation and body condition scoring of donkeys. Centre for Tropical Veterinary Medicine, University of Edinburgh, p 21
Piccione G, Caola G, Refinetti R (2002) The circadian rhythm of body temperature of the horse. Biol Rhythm Res 33(1):113–119
Piccione G, Caola G, Refinetti R (2005) Temporal relationships of 21 physiological variables in horse and sheep. Comp Biochem Physiol A Mol Integr Physiol 142(4):389–396
Piccione G, Grasso F, Fazio F, Giudice E (2008) The effect of physical exercise on the daily rhythm of platelet aggregation and body temperature in horses. Vet J 176:216–220
Piccione G, Giannetto C, Marafioti S, Casella S, Assenza A, Fazio F (2011) Comparison of daily rhythm of rectal and auricular temperatures in horses kept under a natural photoperiod and constant darkness. J Therm Biol 36(4):245–249
Purswell JL, Gates RS, Lawrence LM, Davis JD (2010) Thermal environment in a four-horse slant-load trailer. Trans ASABE 53(6):1885–1894
Pusnik I, Miklavec A (2009) Dilemmas in measurement of human body temperature. Instrum Sci Technol 37:516–530
Ramey D, Bachmann K, Lee ML (2011) A comparative study of non-contact infrared and digital rectal thermometer measurements of body temperature in the horse. J Equine Vet Sci 31(4):191–193
Redaelli V, Luzi F, Mazzola S, Bariffi GD, Zappaterra M, Nanni Costa L, Padalino B (2019) The use of infrared thermography (IRT) as stress indicator in horses trained for endurance: a pilot study. Animals 9(3):84. https://doi.org/10.3390/ani9030084
Robertson JK, Mastromonaco G, Burness G (2020) Evidence that stress-induced changes in surface temperature serve a thermoregulatory function. J Exp Biol 223(4):jeb213421. https://doi.org/10.1242/jeb.213421
Schmidt K, Deichsel K, de Oliveira RA, Aurich J, Ille N, Aurich C (2017) Effects of environmental temperature and season on hair coat characteristics, physiologic and reproductive parameters in Shetland pony stallions. Theriogenology 97:170–178
Schroter RC, Marlin DJ, Jeffcott LB (1996) Use of the wet-bulb globe temperature (WBGT) index to quantity environmental heat loads during three-day-event competitions. Equine Vet J 22:3–6
Sellier N, Guettier E, Staub C (2014) A review of methods to measure animal body temperature in precision farming. Am J Agric Sci Technol 2:74–99
Shechtman O (2013) The coefficient of variation as an index of measurement reliability. In: Doi SAR, Williams GM (eds). Methods of Clinical Epidemiology. Springer, Berlin, Heidelberg, pp 39–49
Sinkalu VO, Ayo JO, Minka NS, Umekesiobi JN (2017) Circadian variations in rectal temperature responses of packed donkeys deprived of feed and water administered with ascorbic acid during the cold-dry (harmattan) season. In 21st International Congress of Biometeorology, September 3-6. Durham University, Durham, p 95
Soroko M, Howell K, Zwyrzykowska A, Dudek K, Zielińska P, Kupczyński R (2016) Maximum eye temperature in the assessment of training in race-horses: correlations with salivary cortisol concentration, rectal temperature, and heart rate. J Equine Vet Sci 45:39–45
Sousa MG, Carareto R, Pereira-Junior VA (2013) Agreement between auricular and rectal measurements of body temperature in healthy cats. J Feline Med Surg 15:275–279
Southward ES, Mann FA, Dodam J (2006) A comparison of auricular, rectal and pulmonary artery thermometry in dogs with anaesthesia-induced hypothermia. J Vet Emerg Crit Care (San Antonio) 16:172–175
Stewart M, Webster JR, Verkerk GA, Schaefer AL, Colyn JJ, Stafford KJ (2007) Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol Behav 92(3):520–525
Stieler AL, Sanchez LC, Mallicote MF, Martabano BB, Burrow JA, MacKay RJ (2016) Macrolide-induced hyperthermia in foals: role of impaired sweat responses. Equine Vet J 48(5):590–594
Suthar V, Burfeind O, Maeder B, Heuwieser W (2013) Agreement between rectal and vaginal temperature measured with temperature loggers in dairy cows. J Dairy Res 80:240–245
Sunday JM, Bates AE, Dulvy NK (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc R Soc B Biol Sci 278:1823–1830
Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240:1285–1293
Takahashi Y, Ohmura H, Mukai K, Shiose T, Takahashi T (2020) A comparison of 5 cooling methods in hot and humid environments in thoroughbred horses. J Equine Vet Sci 91:103130
Thom EC (1959) The discomfort index. Weatherwise 12(2):57–61
Valera M, Bartolomé E, Sánchez MJ, Molina A, Cook N, Schaefer AL (2012) Changes in eye temperature and stress assessment in horses during show jumping competitions. J Equine Vet Sci 32:827–830
Vickers LA, Burfeind O, Von Keyserlingk MAG, Veira DM, Weary DM, Heuwieser W (2010) Comparison of rectal and vaginal temperatures in lactating dairy cows. J Dairy Sci 93(11):5246–5251
Vilela A (2019) Working together to ‘Stamp Out Strangles’. Equine Health 48:24–27
Wenz JR, Moore DA, Kasimanickam R (2011) Factors associated with the rectal temperature of Holstein dairy cows during the first 10 days in milk. J Dairy Sci 94:1864–1872
Wong D (2011) Equine influenza: a clinical perspective in Centennial Parklands Equestrian Centre. Aust Vet J 89:15–16
Xu X, Karis AJ, Buller MJ, Santee WR (2013) Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur J Appl Physiol 113(9):2381–2389
Yang L, Wang W, Huang S, Wang Y, Wronski T, Deng H, Lu J (2019) Individual stress responses of white rhinoceros (Ceratotherium simum) to transport: implication for a differential management. Glob Ecol Conserv 17:e00588. https://doi.org/10.1016/j.gecco.2019.e00588
Zou KH, O’Malley AJ, Mauri L (2007) Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 115:654–657. https://doi.org/10.1161/CIRCULATIONAHA.105.594929
Acknowledgements
The authors thank the technical staff of the National Animal Production Research Institute, Ahmadu Bello University, Shika-Zaria, Nigeria, for their technical support.
Funding
Research was funded by the authors involved in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zakari, F.O., Ayo, J.O. Comparison of body temperature in donkeys using rectal digital, infrared, and mercury-in-glass thermometers during the hot-dry season in a tropical savannah. Int J Biometeorol 65, 1053–1067 (2021). https://doi.org/10.1007/s00484-021-02087-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-021-02087-z