Abstract
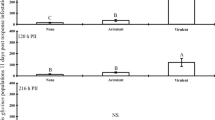
Plants are attacked by a variety of herbivores and respond with localized or systemic defenses. Plant defense against one feeding guild (e.g. chewing, piercing/sucking) may also affect alternate feeding guilds. To optimize host plant resistance, interactions between feeding guilds must be understood. Aphids are plant virus vectors whose vector efficiency can be altered by plant defenses. To determine if systemic induction influences aphid feeding behaviors related to virus transmission, three soybean varieties, Davis, Lyon, and Progeny 4906RR, were induced by subjecting plants to soybean looper (SBL) herbivory, or exogenous applications of either jasmonic acid (JA) or salicylic acid (SA). Green peach aphid (GPA) apterae feeding behavior was recorded on induced and control plants using the Electrical Penetration Graph (EPG) technique. SBL growth bioassays were used to assess systemic induction. Previous SBL herbivory reduced SBL larval weights when fed Progeny 4906RR. JA reduced larval weights on Progeny 4906RR and Davis. SA increased SBL larval weights on Lyon. SBL herbivory decreased behaviors associated with nonpersistent virus transmission in Davis and Progeny 4906RR. JA altered behaviors associated with virus transmission in Davis and increased behaviors associated with virus acquisition in Progeny 4906RR. SA delayed probing in Davis, but increased behaviors associated with virus transmission in Progeny 4906RR and Lyon. Inducing host plant resistance with JA may reduce herbivore performance and increase nonpersistent virus transmission. Previous chewing herbivory may decrease nonpersistent virus transmission by aphids but is variety dependent.



Similar content being viewed by others
References
Accamando AK, Cronin JT (2012) Costs and benefits of jasmonic acid induced responses in soybean. Environ Entomol 41:551–561
Anderson KE, Inouye BD, Underwood N (2015) Can inducible resistance in plants cause herbivore aggregations? Spatial patterns in an inducible plant/herbivore model. Ecology 96:2758–2770
Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defenses. Biochem Biophys Acta 1734:91–111
Baldwin IT (1999) Functional interactions in the use of direct and indirect defences in native Nicotiana plants. In: Chadwick J, Goode JA (eds) Insect-plant interactions and induced plant defense. Wiley, New York, pp 74–94
Boughton AJ, Hoover K, Felton GW (2006) Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphids, Myzus persicae. Entomol Exp Appl 120:175–188
Cao H, Wang S, Liu T (2013) Jasmonate- and salicylate-induced defenses in wheat affect host preference and probing behavior, but not performance of the grain aphid Sitibion avenae. Insect Sci 00:1–9
Caviness CE, Walter HJ (1966) Registration of ‘Davis’ soybean. Crop Sci 6:502
Chen M-S (2008) Inducible direct plant defenses against herbivores: a review. Insect Sci 15:101–114
Collar JL, Fereres A (1998) Nonpersistent virus transmission efficiency determined by aphid probing behavior during intercellular punctures. Environ Entomol 27:583–591
Collar JL, Avilla C, Fereres A (1997) New correlations between aphid stylet paths and nonpersistent virus transmission. Environ Entomol 26:537–544
Coppola V, Soler R, Rao R, Corrado G (2017) Tomato-mediated interactions between root herbivores and aphids: insights into plant defense signaling. Entomol Exp Appl 163:170–176
Davis JA, Radcliffe EB (2008) Reproduction and feeding behavior of Myzus persicae on four cereals. J Econ Entomol 101:9–16
Ellis C, Karafyllidis I, Turner JG (2002) Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact 15:1025–1030
Fehr WR, Caviness CE (1977) Stages of soybean development. Special report 87. http://lib.dr.iastate.edu/specialreports/87
Felton GW, Eichenseer H (1999) Herbivore saliva and its effect on plant defence against herbivores and pathogens. In: Agrawal A, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores. The American Phytopathological Society Press, St. Paul, pp 19–36
Felton GW, Summers CB, Muwller AJ (1994) Oxidative response in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J Chem Ecol 20:639–650
Fereres A, Moreno A (2009) Behavioral aspects influencing plant virus transmission by homopteran insects. Virus Res 141:158–168
Ferry N, Edwards MG, Gatehouse JA, Gatehouse AMR (2004) Plant-insect interactions: molecular approaches to insect resistance. Curr Opin Biotechnol 15:155–161
Gordy JW (2013) A survey of chemical elicitors and their effectiveness as promoters of plant defense against herbivory by Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) M.S. thesis, Louisiana State University
Hartwig EE, Kilen TC, Young LD (1994) Registration of ‘Lyon’ soybean. Crop Sci 34:1412
Hatchett JH, Beland GH, Hartwig EE (1976) Leaf-feeding resistance to bollworm and tobacco budworm in three soybean plant introductions. Crop Sci 16(2):277–280
Heidel AJ, Baldwin IT (2004) Microarray analysis of salicylic acid- and jasmonic acid-signaling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant Cell Environ 27:1362–2137
Heil M, Bueno JCS (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci 104:5467–5472
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66
Kant MR, Jonckheere W, Knegt B et al (2015) Mechanisms and ecological consequences of plant defense induction and suppression in herbivore communities. Ann Bot 115:1015–1051
Katis NI, Tsitsipis JA, Stevens M, Powell G (2007) Transmission of plant viruses. In: Van Emden HF, Harrington R (eds) Aphids as crop pests, 1st edn. CABI, Wallingford, pp 87–113
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Michaud JP, Zhang Y, Bain C (2017) Feeding by Melanaphis sacchari (Hemiptera:Aphididae) facilitates use of sorghum by Rhopalisiphum padi (Hemiptera:Aphididae), but reciprocal effects are negative. Environ Entomol 42:268–273
Mitchell C, Breenan RM, Graham J, Karley AJ (2016) plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop production. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01132/full
Montllor CB, Tjalling WF (1989) Stylet penetration by two aphid species on susceptible and resistant lettuce. Entomol Exp Appl 52:103–111
Morell K, Kessler A (2017) Plant communication in a widespread goldenrod: keeping herbivores on the move. Funct Ecol 31:1049–1061
Musser RO, Cipollini DF, Hum-Musser SM, Williams SA, Brown JK, Felton GW (2005) Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants. Arch Insect Biochem Physiol 58:128–137
Ng JCK, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Pickett JA, Chamberlain K, Poppy GM, Woodcock CM (1999) Exploiting insect responses in identifying plant signals. In: Chadwick J, Goode JA (eds) Insect-plant interactions and induced plant defense. Wiley, New York, pp 253–265
Poehlman JM (1987) Breeding soybeans. In: Poehlman JM (ed) Breeding field crops, 3rd edn. Van Nostrand Reinhold, New York, pp 421–450
Powell G (2005) Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J Gen Virol 86:469–472
Powell G, Pirone T, Hardie J (1995) Aphid stylet activities during potyvirus acquisition from plants and an in vitro system that correlate with subsequent transmission. Eur J Plant Pathol 101:411–420
Prado E, Tjallingii WF (1997) Effects of previous plant infestation on sieve element acceptance by two aphids. Entomol Exp Appl 82:189–200
Selig P, Keough S, Nalam VJ, Nachappa P (2016) Jasmonate-dependent plant defenses mediate soybean thrips and soybean aphid performance on soybean. Arthropod Plant Interact 10:273–282
Smith CM, Boyko EV (2006) The molecular basis of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl 122:1–16
Smith CM, Clement SL (2012) Molecular basis of plant resistance to arthropods. Annu Rev Entomol 57:309–328
Srinivas P, Danielson SD (2001) Effect of the chemical inducer ActigardTM in inducing resistance to bean leaf beetle, Cerotoma trifurcate (Forster) (Coleoptera: Chysomelidae), feeding in soybean. J Agric Urban Entomol 18:209–215
Stout M, Davis J (2009) Keys to the increased use of host plant resistance in integrated pest management. In: Peshin R, Dhawan AK (eds) Integrated pest management: innovation-development process. Springer, Berlin, pp 163–180
Stout MJ, Workman KV, Bostock RM, Duffey SS (1998) Specificity of induced resistance in the tomato, Lycopersicon esculentum. Oecologia 113:74–81
Tan XL, Chen JL, Benelli G, Desneux N, Yang XQ, Liu TX, Ge F (2017) Pre-infestation of tomato plants by aphids modulates transmission-acquisition relationship among whiteflies, tomato yellow leaf curl virus (TYLCV) and plants. Front Plant Sci 8:1–11
Underwood NC (1998) The timing of induced resistance and induced susceptibility in the soybean-Mexican bean beetle system. Oecologia 114:376–381
Underwood NC, Morris W, Gross K, Lockwood JR III (2000) Induced resistance to Mexican bean beetle in soybean: variation among genotype and lack of correlation with constitutive resistance. Oecologia 122:83–89
Underwood NC, Rausher M, Cook W (2002) Bioassay versus chemical assay: measuring the impact of induced and constitutive resistance on herbivores in the field. Oecologia 131:211–219
Van der Does D, Leon-Reyes A, Koornneef A et al (2013) Salicylic acid suppressed jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25:744–761
Vos IA, Pieterse CMJ, van Wees SCM (2013) Costs and benefits of hormone-regulated plant defense. Plant Path 62:43–55
Walker GP (2000) A beginner’s guide to electronic monitoring of homopteran feeding behavior. In: Walker GP, Backus EA (eds) Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Entomological Society of America, Lanham, pp 14–40
Wosula EN, Clark CA, Davis JA (2012) Effect of host plant, aphid species, and virus infection status on transmission of Sweetpotato feathery mottle virus. Plant Dis 96:1331–1336
Xuan C, Richter AR, Stout MJ, Davis JA (2018) Effects of induced plant resistance on soybean looper (Lepidoptera: Noctuidae) in soybean. Arthropod Plant Interact 13:543–551
Zhang Y, Fan J, Francis F, Chen J (2017) Watery saliva secreted by the grain aphid Sitbion avenae stimulates aphid resistance in wheat. J Agric Food Chem 65:8798–8805
Züst T, Agrawal AA (2017) Trade-offs between plant growth and defense against insect herbivory: an emerging mechanical synthesis. Annu Rev Plant Biol 68:513–534
Acknowledgements
This article was approved for publication by the Director of the Louisiana Agricultural Experiment Station as manuscript No. 2020-234-34838.
Funding
This project was partially funded by the Louisiana Soybean and Grain Research and Promotion Board and by the Louisiana Agricultural Experiment Station.
Author information
Authors and Affiliations
Contributions
J.L.D. and J.A.D. designed the study. J.L.D. performed the experiments and analyzed the data. J.L.D. and J.A.D. wrote the manuscript and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing financial interests.
Additional information
Handling Editor: Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dryburgh, J.L., Davis, J.A. Effect of soybean variety and systemic induction on herbivore feeding guilds. Arthropod-Plant Interactions 15, 171–181 (2021). https://doi.org/10.1007/s11829-021-09806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09806-8