Abstract
Surface hydrophobicity/hydrophilicity of titania (TiO2) films, spin-coated on silicon wafers, were tuned by introducing surface mesopores with various morphologies using a triblock copolymer F38 as the template agent of different weight ratios via a sol-gel method. It is found that both the porosity (2.92 ∼ 33.03%) and the surface roughness (0.22 ∼ 0.43 nm for arithmetic mean roughness and 0.28 ∼ 0.58 nm for root mean square roughness) of the films increase monotonically as increasing F38 ratio from 5 to 25 wt%, accompanied by distinct changes of pore morphology from isolated mesopores with pore sizes of 5 ∼ 7 nm to longer worm-like pores (30 ∼ 100 nm in length). The apparent static contact angle (θ*) of the films with isolated mesopores is enhanced from ca. 90.6° to 100.1° as indicated by an increase of the roughness factor with incresing F38 from 5 to 15 wt%, which is in qualitative agreement with the Wenzel's equation. Interestingly, the films with interconnected worm-like pores show obvious hydrophilicity (θ* = 80.7°) with further increasing F38 ratio higher than 20 wt%. The reversed surface wettability show that not only surface roughness but also pore morphology could significantly affect the wettability of the mesoporous TiO2 films.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Environment-friendly, biocompatible, economic, and recyclable mesoporous titania (namely titanium dioxide, TiO2) is one of the most popular semiconducting metal dioxide, due to its potential applications in solar cells [1, 2], chemical sensors [3, 4], and photocatalysts [5, 6]. Surface wetting properties of TiO2-based functional devices play an important role in many practical applications. Therefore, the wettability behaviors on the TiO2 or TiO2-based surfaces, such as superhydrophobic surface [7], superhydrophilic surface [8], and wettability switching [9–11], have attracted worldwide research interests. It is generally accepted that the wetting properties of a solid surface greatly depends on its surface roughness (geometrical structure) and surface energy (the variation of chemical compositions) [10–12]. Among the efforts to develop TiO2 films with different wetting surfaces, introduction of pores into TiO2 films is probably a much convenient and controllable method owing to its easy operation.
Great efforts have been made for accurately controlling [13–17] the porous structures of mesoporous thin films, which is also one of the most promising research focuses for improvement of mesostructured TiO2 or expansion of specific application scope. The successful synthesis of a family of silicate/aluminosilicate mesoporous molecular sieve named M41S [18], provides a new idea for the preparation of templated mesoporous structures. For instance, amphiphilic block copolymers [19–22] have been introduced for guiding the synthesis of groups of mesoporous films with adjustable pore sizes and controllable morphologies. The method benefits from the self-assembly ability of amphiphilic copolymers to minimize their surface energy in solutions [23]. By mixing organic structural template and inorganic precursors, they are co-assembled into a so-called 'titanotropic' mesophase [24–26], and ultimately mesoporous structures are formed after removal of the template. For this synthetic method, the additive content is critical for the determination of mesoporous structures. Such copolymer-templated mesostructures overcome the limitations of dimensionality, making well-developed pores readily available, thus arousing great interests in various application fields. The introduction of pore structures into a TiO2 film makes its surface possess physical heterogeneity. Hence, deep understanding of the effect of such a heterogenous structure on surface wettability is of importance.
In this work, porous TiO2 films were prepared via above mentioned sol-gel method in the presence of copolymer Pluronic F38 with various weight ratios as the structure-directing agent. The mesostructures of the TiO2 films, such as porosity, pore morphology and surface roughness, were studied by ellipsometry, field emission scanning electron microscopy (FE-SEM) and atomic force microscopy (AFM). The effects of surface roughness and pore morphology on surface wettability quantified by static contact angle were discussed. The results might contribute to deeper understandings of the cooperative assembly between copolymer and precursor, and the dependence of wettability of porous films on their mesostructures.
2. Experimental
2.1. Sample preparation
Titanium tetrachloride (TiCl4) and copolymer F38 (EO43PO14EO43, Mw = 4700 g mol−1, a BASF surfactant) were chosen as the titania source and structural template, respectively. The titanium sols were synthesized via a simple sol-gel route as previously reported [27] by introducing various weight ratios of F38, which were designated as 5, 10, 15, 20 and 25 wt%, respectively, calculated as WF38/(WF38 + WTiCl4). The mole ratios of the final compositions in the titanium sols were 1 TiCl4: x F38: 25 EtOH (anhydrous ethanol): 4 H2O: 0.002 HCl, where x was in the range of 0 to 0.013 depending on the weight ratio of F38. The resulting sols were subsequently deposited on monocrystalline Si (100) substrates by spin-coating. After being dried in a vacuum at 100 °C for 2 h, the films were calcined at 500 °C for 4 h under pure nitrogen gas flow to decompose the polymeric template.
2.2. Characterization of titania films
An ellipsometer (α-SE, J. A. Woollam Co., Inc. Ellipsometry Solution) was applied to measure the refractive indices (n) of the TiO2 films under nitrogen atmosphere. All characteristic n values at 632.8 nm were calculated by fitting ellipsometric parameters ∆ and Ψ in the wavelength range of 380 ∼ 900 nm on the basis of the Cauchy model [28]. The refractive indices of six different spots on each film sample were measured and averaged. The mean thicknesses of the TiO2 films obtained from ellipsometry measurements are 213.5, 119.7, 230.7, 252.0, 343.7 nm, respectively. The morphologies of the TiO2 film surfaces were evaluated by AFM observations performed by a SHIMADZU SPM-9500J3 instrument. The scanning area of the TiO2 film surfaces was 1 μm × 1 μm. The static contact angle was used to quantify the wettability of porous TiO2 films. A goniometer (Minilab, ILMS, France) was operated to determine the static contact angle by dropping a droplet of deionized water in a volume of ca. 1 μl onto the TiO2 film surface with a micro-syringe. For each TiO2 film, six individual droplets were correspondingly placed on six different locations.
3. Results and discussion
3.1. Porosity and pore morphology of titania films
Hydrophobic polypropylene oxide (PPO) blocks and hydrophilic polyethylene oxide (PEO) blocks coexist in the triblock copolymer F38. Micellar structures are usually formed in solutions by the microphase separation of such amphiphilic copolymers. The cores of the micelles are the aggregated PPO chains, which surrounded by the coronal PEO chains [29]. Upon the addition of titanium precursor, titanium species selectively swells in the hydrophilic PEO chains of the copolymer. The resulted complexes self-assemble into a 'titanotropic' mesophase [24–26], accompanied by the hydrolytic polymerization of the inorganic species. Pores are generated in TiO2 films after removal of the surfactant F38 upon calcination at a high temperature. Thus, mesostructured TiO2 may be tuned by varying the weight ratios of triblock copolymer F38.
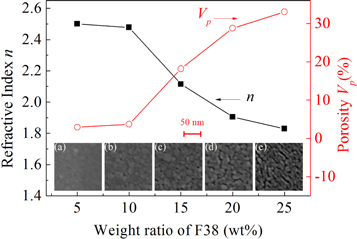
The effect of F38 loading on the refractive indices of the TiO2 films is depicted in figure 1. For the TiO2 film fabricated with 5 wt% F38, the refractive index is around 2.50, slightly less than that of nonporous anatase TiO2 (2.59) [30]. As the amount of F38 increases from 5 to 25 wt%, the monotonic reduction in n of the TiO2 films from 2.50 to 1.83 reveals the increment in porosity by introducing more F38. The porosities (Vp ) of the porous TiO2 films can be calculated by the simplified Lorentz-Lorenz formula as follows [31],
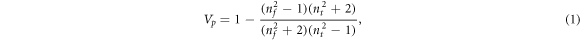
where the subscript f and t represent the TiO2 film and TiO2 network skeleton, respectively. In this study, the refractive index of TiO2 crystals in anatase phase (2.59) is used for the value of nt in the above equation. The dependence of Vp of the porous TiO2 films on F38 content is also displayed in figure 1. A lower refractive index corresponds to a higher porosity for the films. It's rational to find that the porosity of the templated porous TiO2 films rises monotonically from 2.92% to 33.03% with loading sacrificed porogen F38 from 5 to 25 wt%.
Figure 1. The refractive indices (solid squares) and porosities (open circles) of the porous TiO2 films as a function of F38 weight ratio. The insets are the FE-SEM images of TiO2 films with various weight ratios of F38: (a) 5 wt%, (b) 10 wt%, (c) 15 wt%, (d) 20 wt%, and (e) 25 wt%. (Reprinted with permission from [27]. Copyright 2017 Elsevier).
Download figure:
Standard image High-resolution imageThe pore morphology of the porous TiO2 films has been demonstrated by the FE-SEM images in the insets of figure 1, which was also shown in our previous paper [27]. No evident pores are present in the TiO2 film prepared by adding 5 wt% of F38. Isolated, small pores with pore size of 5 ∼ 7 nm are found in the TiO2 films templated by 10 wt% and 15 wt% F38. Interestingly, the interconnection of mesopores emerges when the additive loading is as high as 20 wt% and worm-shaped pores in length of 30 ∼ 80 nm show up. Higher pore interconnectivity and longer 'worms' (40 ∼ 100 nm in length) are found as further increasing F38 amount to 25 wt%. The dependence of pore structure of the porous silica films on the amount of the same porogen was reported previously, indicative of that the mesopore percolation occurred around the F38 amount of 10 wt% [32].
3.2. Surface structure of titania films
Figure 2 depicts the surface morphologies of the porous TiO2 films synthesized by various loadings of F38. The maximum height of the TiO2 surfaces grows gradually from 2.33 to 3.97 nm with increasing template amount from 5 to 15 wt%, and then decreases to 3.18 nm for the TiO2 film prepared by adding 20 wt% of F38. As F38 content further increases to 25 wt%, the maximum height distinctly increases to 5.64 nm. The surface roughness of the TiO2 films is quantified by arithmetic mean roughness (Ra ) and root mean square roughness (RMS), which can be estimated by the following formulas [33, 34].
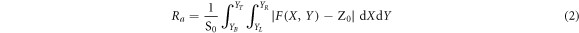
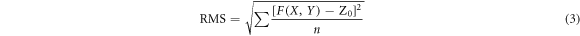
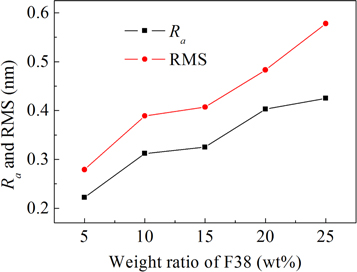
Here S0 stands for the designated area of the film surface, F (X, Y) represents the height at position (X, Y), and Z0 denotes the mean value of F (X, Y) over the designated area. Figure 3 displays Ra and RMS of the porous TiO2 films evaluated in this way from AFM data as a function of F38 loading. As the addition of F38 increases from 5 to 25 wt%, Ra value increases monotonically from ca. 0.22 nm to 0.43 nm, indicating that more and larger pores are generated in the TiO2 films by adding more F38. The variation of RMS as a function of F38 content has the same trend with that of Ra . RMS grows monotonically from ca. 0.28 to 0.58 nm by loading F38 from 5 to 25 wt%. The AFM observations also indicated the increase of pore size/concentration by introducing more F38. Interestingly, for the films introduced F38 loading greater than 15 wt%, RMS is more dependent on F38 amount. The more significant increase in the RMS value of the TiO2 films (>15 wt%) can be attributed to the formation of longer interconnected pores with higher porosities. The results agree with those observed by ellipsometry and FE-SEM.
Figure 2. AFM images of the porous TiO2 films prepared with various weight ratios of F38: (a) 5 wt%, (b) 10 wt%, (c) 15 wt%, (d) 20 wt%, and (e) 25 wt%.
Download figure:
Standard image High-resolution imageFigure 3. The dependence of the average roughness (Ra ) and root mean square roughness (RMS) of the porous TiO2 films on the amount of F38.
Download figure:
Standard image High-resolution image3.3. Evolution of mesostructure in titania films
As is well known that thermodynamically stable micelles can be formed in solutions only when the concentration of the triblock copolymer reaches a certain value, which is named critical micellization concentration, abbreviated as CMC [35]. Below the CMC value, the block copolymers exist as unimers. The CMC value of a block copolymer is directly related to its self-assembly ability in the preparation of porous structures [36]. It is often hard to develop highly ordered porous materials by using copolymers with high CMC as the structural templates. It is found that the CMC value of triblock copolymer F38 in EtOH solution is about 1.46 wt%, equivalent to the titanium sol prepared with 10 wt% of F38, which is revealed by the isolated mesopores in the TiO2 film. The result is in good accordance with that of TiO2 powders prepared via the same method, reported in a previous paper [37]. At low concentration of F38 (5 wt%), the self-assembly ability of F38 is too weak to form stable micellar structures in solution. The low porosity of ca. 2.92% is principally resulted from the voids among the TiO2 grains. When the F38 loading exceeds ca. 10 wt%, stable micelles can be formed in sols, thereby forming small and isolated pores in the TiO2 films. The threshold of pore percolation in the TiO2 films is found at F38 ratio of ca. 15 wt%. The random mesopores with higher concentration are connected to each other in the form of 'worms' as F38 loading reaches 20 wt%. The pore interconnectivity is enhanced and worm-like pores are further elongated by introducing F38 amount to 25 wt%.
3.4. Surface wettability of titania films
For a liquid or solid, the surface atoms or molecules are more energetic than its internal ones, which leads to surface free energy or surface tension, thereby reaching a steady state with relatively lower energy [38]. For a water droplet staying on a solid surface, its shape is determined by the pulling force of the droplet at the three-phase contact line on the solid plane, where the interfaces of solid/liquid, gas/solid, and liquid/gas meet. If the droplet is small enough, gravity can be neglected. The droplet will be spherically shaped and the gas/liquid interface will contact the solid surface at an angle θ, which is universally recognized as the inherent contact angle of the droplet. The shape of the droplet on a solid in equilibrium state is determined by the following formula proposed by Thomas Young [39],
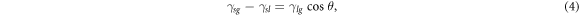
where the subscript s, g, and l represent solid phase, gas phase, and liquid phase, respectively. The symbol γ represents the surface tension between the two of three phases expressed by the subscripts. The Young's formula is the basic law for all the wetting phenomena. For the contact angle less than 90°, the surface is generally considered to be hydrophilic, while the surface with contact angle greater than 90° is conventionally regarded as being hydrophobic.
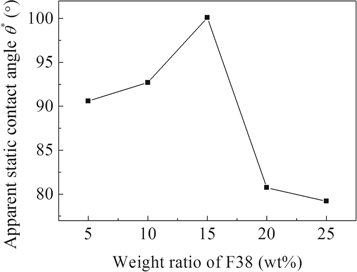
Figure 4 shows the apparent static contact angle (θ*) of the porous TiO2 films prepared by varying F38 loading. For the film prepared with adding 5 wt% F38, θ* is around 90.6°, indicative of a hydrophobic surface. The wettability of the film directed by 10 wt% of F38 also corresponds to hydrophobicity for θ* ≈ 92.7°. The apparent contact angle increases to ca. 100.1° as the weight ratio of F38 rises to 15 wt%. A gradient of surface wettability with enhanced hydrophobicity is fabricated by changing template content from 5 to 15 wt%. However, as the loading amount of F38 reaches 20 wt%, θ* drops to ca. 80.7°, showing a hydrophilic surface. The further reduction in θ* to about 79.2° is observed as the loading of F38 rises to 25 wt%. The surface wettability of the TiO2 films transforms from hydrophobicity to hydrophilicity as the copolymer content increases from 15 to 20 wt%.
Figure 4. The apparent static contact angle θ* of the porous TiO2 films with various weight ratios of F38.
Download figure:
Standard image High-resolution imageThe Young's formula is valid for ideal, smooth and homogeneous surfaces. In practice, the surfaces of the porous TiO2 films are rough and heterogeneous, therefore the basic effects of surface structures should be taken into account. The surface roughness dependent wettability is usually formulated by the Wenzel's equation [40],
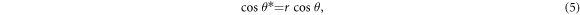
where r is roughness factor, a non-dimensional parameter, defined as the ratio of actual surface area of a rough surface to its planar area. The Wenzel's equation shows that θ* of a droplet on a rough surface is closely correlated with θ on an ideal one. Because of the increment in the actual surface area of the rough surface, r is greater than 1. According to the Wenzel's formula, on one hand, for a hydrophobic surface, θ > 90°, thereby θ* > θ. On the other hand, for a hydrophilic one, θ < 90°, thus θ* < θ. The Wenzel's equation demonstrates that the hydrophobicity of a rough hydrophobic solid is enhanced by roughness factor, so is the hydrophilicity of a rough hydrophilic surface. Thus, the apparent contact angle can be tuned by varying surface roughness of the solid surface. Recently, it has been confirmed that the surface roughness of the solid can greatly enhance the wettability even without considering the chemical composition [38].
In order to elucidate the dependence of wettability on surface roughness of the solid, a simple regular rough surface of TiO2 film is schematically illustrated in figure 5(a). Parameter a is the pore dimension parallel to the film surface. The pore dimension perpendicular to the film denotes pore height (h), and the space between two mesopores is c. Therefore, the roughness factor r of the rough surface can be expressed as
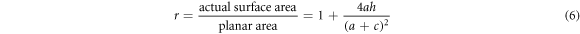
The area of a selected specified region (a + c)2 is constant for all films. From the above equation, the roughness factor of the porous TiO2 films depends on their pore size a and pore height h. The average pore size a varies little in TiO2 films prepared with 10 wt% and 15 wt% F38. Thus, the change of r is mainly attributed to h, and r can be supposed to be proportional to h.
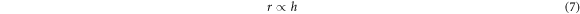
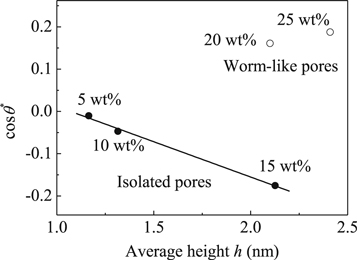
cos θ* as a function of the average pore height (calculated by AFM measurements) of the porous TiO2 films by loading various amount of F38 is plotted in figure 6. The experimental values are well located on a straight fitting-line with the slope of k = −0.167 ± 0.011 for the TiO2 films by introducing lower amount of F38 (≤15 wt%). As the copolymer amount increases from 5 to 15 wt%, cosθ* decreases linearly from ca. −0.010 to −0.175 with the increase of h from 1.165 to 2.126 nm. The Wenzel's equation predicts that cos θ* decreases with increasing r for θ > 90°, which is qualitative consistent with the experimental data in figure 6, for the TiO2 films directed by lower content of template (≤15 wt%). The enhancement of hydrophobicity is resulted from the introduction of greater roughness factor, accompanied by the increase of total surface/interface energy owing to the enlargement of the actual area [33]. However, the tendency of cosθ* on h is unable to continue for the films templated by F38 loading higher than 15 wt%, showing significant deviations from the linear relationship. On the contrary, cos θ* increases remarkably from ca. −0.175 to 0.161 as the weight ratio of F38 increases from 15 to 20 wt%, indicating that the wetting property of the TiO2 films transforms from hydrophobicity to hydrophilicity.
Figure 5. Schematic illustrations of (a) a regular rough surface of porous TiO2 film, (b) droplet of water on a hydrophobic TiO2 film and (c) droplet of water on a hydrophilic TiO2 film.
Download figure:
Standard image High-resolution imageFigure 6. The plots of cos θ* versus the average height h of the porous TiO2 films with various weight ratios of F38. The solid line is a data-fitting based on equations (5) and (7).
Download figure:
Standard image High-resolution imageOne can conclude that the wetting behavior of water on the TiO2 film surfaces depends not only on surface roughness but also on pore morphology based on the Wenzel's theory and experimental results. On one hand, for the rough surfaces with similar pore morphology, the surface roughness plays a significant role in surface wettability. The aspect ratio of a pore here is used to describe its pore morphology semiquantitatively, which is defined as the ratio of pore size to pore height (a/h). For the hydrophobic TiO2 films with isolated pores (≤15 wt%), as described in figure 5(b), a/h is in the range of 2.25 ∼ 6.00, where the experimental results are in good consistent with the rule of Wenzel's equation even for the randomly rough surfaces. Thus, for the TiO2 films consisted of isolated pores with lower aspect ratios, the hydrophobicity is enhanced by the increase of roughness factor.
On the other hand, when the pore morphology changes significantly, the surface wettability is affected predominantly by pore morphology. The variation of h is little (from 2.13 to 2.41 nm) as copolymer loading increases from 15 to 25 wt%, wheareas a increases significantly from several nanometers to several tens nanometers because of the enhancement in the interconnectivity of pores, resulting in the change of pore morphologys from isolated pores to interconnected worm-like pores. The aspect ratio ranges from a few to several tens by introducing copolymer F38 from 15 to 25 wt%. The result is quite in accord with the effect of template F38 loading on the pore size of mesoporous silica films, as reported in a previous paper [32], showing that the development of pores is primarily through one-dimensional prolongation instead of three-dimensional expansion of pore size. For longer worm-like tuble pores (>15 wt%), r is mainly influenced by the elongated pore dimension a. According to equation (6), with the increase of a, the hydrophobicity based on the Wenzel's equation is supposed to be further increased correspondingly as r increases. However, it is not the case for the films with the aspect ratios of pores as high as tens. Despite of much greater r of the films with more F38, the films with longer worm-like pores show amazingly obvious hydrophilicity instead of hydrophobicity, as depicted in figure 5(c). It is worth noting that the Wenzel's equation is also suitable for the hydrophilic films with similar worm-like pores. In other words, the hydrophilicity (from 80.7° to 79.2°) is improved with the increase of roughness factor by adding F38 amount from 20 to 25 wt%.
Generally speaking, owing to the heterogeneity of a rough surface, the liquid often needs to surmount an energy barrier during spreading, and will remain metastable rather than stable when the vibration energy is less than the barrier [33]. The wettability might transform because of the probable coexistence of homogeneous and inhomogeneous wetting states on the same surface where energy barriers are present, resulting in very diffident apparent contact angles. Furthermore, almost all theoretical models on rough surfaces are in a symmetric state simplified as a well-organized patterned solid. However, the surfaces in practice are much more complicated in disordered states. In addition, capillary condensation of water in the well-interconnected pores might be responsible for the hydrophilicity of the surfaces for the films templated by higher loading of F38 (>15 wt%). The capillary enhancement of a hydrophilic surface can be expressed by the Young-Laplace equation [12]:
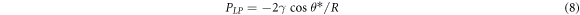
where PLP is the Laplace pressure in a pore channel, γ is the surface tension, and R is the radius of the channel. From equation (8), PLP is governed by the contact angle and the channel radius. For the hydrophilic film surfaces with worm-like pore channels, PLP is negative, so it acts as a driving force when water is introduced. Hence, from the perspective of structure and application, more attention should be paid to the mechanism on such disordered surfaces in future to better realize industrial application, for instance, in precisely mimicking natural superhydrophobic surfaces for self-cleaning.
4. Conclusions
Mesoporous TiO2 films with various porosities were fabricated with the assistance of a BASF surfactant F38 of various weight ratios. The surface hydrophobicity/hydrophilicity of the films was tuned with controllable pore morphologies and surface roughness. The wetting behavior of water on the film surfaces depended not only on the surface roughness but also on the pore morphology. For the TiO2 films with isolated mesopores, the improved hydrophobicity was attributed to the increase of roughness factor by introducing more pores with increasing F38 loading from 5 to 15 wt%. Whereas, as the weight ratio of F38 increased from 15 to 20 wt%, isolated pores with a higher concentration coalesced to longer worm-like tubal pores, resulting in surface wettability transformation from hydrophobicity to hydrophilicity. The present results were in qualitative agreement with the Wenzel's equation, and meaningful for deeper understandings of the water wetting behaviors on porous films with various pore morphology and surface structures.
Acknowlegments
This work was financially supported in part by the National Natural Science Foundation of China (NSFC) under Grants Nos. 11705029 and 11875209, the Natural Science Foundation of Guangdong Province, China (No. 2017A030313038), the Key Project of Department of Education of Guangdong Province (No. 2016GCZ008), and the Project of Engineering Research Center of Foshan (No. 20172010018).