Abstract
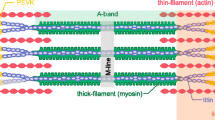
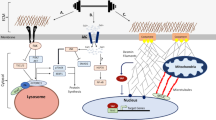
A transduced mechanical signal arriving at its destination in muscle alters sarcomeric structure and function. A major question addressed is how muscle mass and tension generation are optimized to match actual performance demands so that little energy is wasted. Three cases for improved energy efficiency are examined: the troponin complex for tuning force production, control of the myosin heads in a resting state, and the Z-disc proteins for sarcomere assembly. On arrival, the regulation of protein complexes is often controlled by post-translational modification (PTM), of which the most common are phosphorylation by kinases, deacetylation by histone deacetylases and ubiquitination by E3 ligases. Another branch of signals acts not through peptide covalent bonding but via ligand interactions (e.g. Ca2+ and phosphoinositide binding). The myosin head and the regulation of its binding to actin by the troponin complex is the best and earliest example of signal destinations that modify myofibrillar contractility. PTMs in the troponin complex regulate both the efficiency of the contractile function to match physiologic demand for work, and muscle mass via protein degradation. The regulation of sarcomere assembly by integration of incoming signaling pathways causing the same PTMs or ligand binding are discussed in response to mechanical loading and unloading by the Z-disc proteins CapZ, α-actinin, telethonin, titin N-termini, and others. Many human mutations that lead to cardiomyopathy and heart disease occur in the proteins discussed above, which often occur at their PTM or ligand binding sites.


Similar content being viewed by others
References
Al-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP (2013) Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci USA 110:318–323. https://doi.org/10.1073/pnas.1212708110
Alamo L et al (2016) Conserved intramolecular interactions maintain myosin interacting-heads motifs explaining tarantula muscle super-relaxed state structural basis. J Mol Biol 428:1142–1164. https://doi.org/10.1016/j.jmb.2016.01.027
Bárány M (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50(Suppl):197–218. https://doi.org/10.1085/jgp.50.6.197
Bloch RJ, Gonzalez-Serratos H (2003) Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev 31:73–78. https://doi.org/10.1097/00003677-200304000-00004
Bos JM et al (2006) Genotype-phenotype relationships involving hypertrophic cardiomyopathy-associated mutations in titin, muscle LIM protein, and telethonin. Mol Genet Metab 88:78–85. https://doi.org/10.1016/j.ymgme.2005.10.008
Broughton KM et al (2016) A myosin activator improves actin assembly and sarcomere function of human-induced pluripotent stem cell-derived cardiomyocytes with a troponin T point mutation. Am J Physiol Heart Circ Physiol 311:H107-117. https://doi.org/10.1152/ajpheart.00162.2016
Burgoyne T, Morris EP, Luther PK (2015) Three-dimensional structure of vertebrate muscle z-band: the small-square lattice Z-band in rat cardiac muscle. J Mol Biol 427:3527–3537. https://doi.org/10.1016/j.jmb.2015.08.018
Burgoyne T et al (2019) Three-dimensional structure of the basketweave Z-band in midshipman fish sonic muscle. Proc Natl Acad Sci USA 116:15534–15539. https://doi.org/10.1073/pnas.1902235116
Cai XL et al (2019) Cardiac MLC2 kinase is localized to the Z-disc and interacts with α-actinin2. Sci Rep 9:12580. https://doi.org/10.1038/s41598-019-48884-w
Caldwell JE, Heiss SG, Mermall V, Cooper JA (1989) Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28:8506–8514. https://doi.org/10.1021/bi00447a036
Candasamy AJ et al (2014) Phosphoregulation of the titin-cap protein telethonin in cardiac myocytes. J Biol Chem 289:1282–1293. https://doi.org/10.1074/jbc.M113.479030
Carballo S et al (2009) Identification and functional characterization of cardiac troponin I as a novel disease gene in autosomal dominant dilated cardiomyopathy. Circ Res 105:375–382. https://doi.org/10.1161/circresaha.109.196055
Chan JY et al (2008) Identification of cardiac-specific myosin light chain kinase. Circ Res 102:571–580. https://doi.org/10.1161/circresaha.107.161687
Chang AN et al (2015) Constitutive phosphorylation of cardiac myosin regulatory light chainin vivo. J Biol Chem 290:10703–10716. https://doi.org/10.1074/jbc.M115.642165
Chiu C et al (2010) Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J Am Coll Cardiol 55:1127–1135. https://doi.org/10.1016/j.jacc.2009.11.016
Choudhary C et al (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325:834–840. https://doi.org/10.1126/science.1175371
Chowrashi P, Mittal B, Sanger JM, Sanger JW (2002) Amorphin is phosphorylase; phosphorylase is an alpha-actinin-binding protein. Cell Motil Cytoskelet 53:125–135. https://doi.org/10.1002/cm.10059
Chu M, Gregorio CC, Pappas CT (2016) Nebulin, a multi-functional giant. J Exp Biol 219:146–152. https://doi.org/10.1242/jeb.126383
Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL (2010) Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 588:981–993. https://doi.org/10.1113/jphysiol.2009.183897
Cooper JA, Pollard TD (1985) Effect of capping protein on the kinetics of actin polymerization. Biochemistry 24:793–799. https://doi.org/10.1021/bi00324a039
Dai Y et al (2020) Troponin destabilization impairs sarcomere-cytoskeleton interactions in iPSC-derived cardiomyocytes from dilated cardiomyopathy patients. Sci Rep 10:209. https://doi.org/10.1038/s41598-019-56597-3
Disatnik MH, Buraggi G, Mochly-Rosen D (1994) Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res 210:287–297. https://doi.org/10.1006/excr.1994.1041
Duan G, Walther D (2015) The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput Biol 11:e1004049. https://doi.org/10.1371/journal.pcbi.1004049
Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA (2014) Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol 15:677–689. https://doi.org/10.1038/nrm3869
Ehler E (2018) Actin-associated proteins and cardiomyopathy-the “unknown” beyond troponin and tropomyosin. Biophys Rev 10:1121–1128. https://doi.org/10.1007/s12551-018-0428-1
Eisenberg BR, Salmons S (1981) The reorganization of subcellular structure in muscle undergoing fast-to-slow type transformation. A stereological study. Cell Tissue Res 220:449–471. https://doi.org/10.1007/bf00216750
Eisenberg BR, Brown JMC, Salmons S (1984) Restoration of fast muscle characteristics following cessation of chronic stimulation. Cell Tissue Res 238:221–230. https://doi.org/10.1007/BF00217292
Foster DB, Liu T, Rucker J, O’Meally RN, Devine LR, Cole RN, O’Rourke B (2013) The cardiac acetyl-lysine proteome. PLoS ONE 8:e67513. https://doi.org/10.1371/journal.pone.0067513
Fraley TS, Tran TC, Corgan AM, Nash CA, Hao J, Critchley DR, Greenwood JA (2003) Phosphoinositide binding inhibits alpha-actinin bundling activity. J Biol Chem 278:24039–24045. https://doi.org/10.1074/jbc.M213288200
Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T (1992) Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature 359:150–152. https://doi.org/10.1038/359150a0
Fukami K, Sawada N, Endo T, Takenawa T (1996) Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle alpha-actinin. J Biol Chem 271:2646–2650. https://doi.org/10.1074/jbc.271.5.2646
Gautel M, Djinović-Carugo K (2016) The sarcomeric cytoskeleton: from molecules to motion. J Exp Biol 219:135–145. https://doi.org/10.1242/jeb.124941
Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG (1990) A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 62:999–1006. https://doi.org/10.1016/0092-8674(90)90274-i
Gibbs CL (2003) Cardiac energetics: sense and nonsense. Clin Exp Pharmacol Physiol 30:598–603. https://doi.org/10.1046/j.1440-1681.2003.03878.x
Goldstein MA, Michael LH, Schroeter JP, Sass RL (1989) Two structural states of Z-bands in cardiac muscle. Am J Physiol 256:H552-559. https://doi.org/10.1152/ajpheart.1989.256.2.H552
Gomes AV, Harada K, Potter JD (2005) A mutation in the N-terminus of troponin I that is associated with hypertrophic cardiomyopathy affects the Ca(2+)-sensitivity, phosphorylation kinetics and proteolytic susceptibility of troponin. J Mol Cell Cardiol 39:754–765. https://doi.org/10.1016/j.yjmcc.2005.05.013
Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B (2009) CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am J Physiol Cell Physiol 296:C1034-1039. https://doi.org/10.1152/ajpcell.00544.2008
Henze M et al (2013) New insights into the functional significance of the acidic region of the unique N-terminal extension of cardiac troponin I. Biochim Biophys Acta (BBA) Mol Cell Res 1833:823–832. https://doi.org/10.1016/j.bbamcr.2012.08.012
High CW, Stull JT (1980) Phosphorylation of myosin in perfused rabbit and rat hearts. Am J Physiol 239:H756-764. https://doi.org/10.1152/ajpheart.1980.239.6.H756
Hinken AC, Hanft LM, Scruggs SB, Sadayappan S, Robbins J, Solaro RJ, McDonald KS (2012) Protein kinase C depresses cardiac myocyte power output and attenuates myofilament responses induced by protein kinase A. J Muscle Res Cell Motil 33:439–448. https://doi.org/10.1007/s10974-012-9294-9
Hoshijima M (2006) Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol 290:H1313-1325. https://doi.org/10.1152/ajpheart.00816.2005
Jeong MY et al (2018) Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao0144
Kamm KE, Stull JT (2011) Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem 286:9941–9947. https://doi.org/10.1074/jbc.R110.198697
Kedar V, McDonough H, Arya R, Li H-H, Rockman HA, Patterson C (2004) Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA 101:18135–18140. https://doi.org/10.1073/pnas.0404341102
Kellermayer D, Smith JE 3rd, Granzier H (2019) Titin mutations and muscle disease. Pflugers Arch 471:673–682. https://doi.org/10.1007/s00424-019-02272-5
Kim T, Cooper JA, Sept D (2010) The interaction of capping protein with the barbed end of the actin filament. J Mol Biol 404:794–802. https://doi.org/10.1016/j.jmb.2010.10.017
Kim K, McCully ME, Bhattacharya N, Butler B, Sept D, Cooper JA (2007) Structure/function analysis of the interaction of phosphatidylinositol 4,5-bisphosphate with actin-capping protein: implications for how capping protein binds the actin filament. J Biol Chem 282:5871–5879. https://doi.org/10.1074/jbc.M609850200
Knöll R et al (2011) Telethonin deficiency is associated with maladaptation to biomechanical stress in the mammalian heart. Circ Res 109:758–769. https://doi.org/10.1161/circresaha.111.245787
Kooij V, Boontje N, Zaremba R, Jaquet K, dos Remedios C, Stienen GJ, van der Velden J (2010) Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res Cardiol 105:289–300. https://doi.org/10.1007/s00395-009-0053-z
Kuhn JR, Pollard TD (2007) Single molecule kinetic analysis of actin filament capping. Polyphosphoinositides do not dissociate capping proteins. J Biol Chem 282:28014–28024. https://doi.org/10.1074/jbc.M705287200
Kumar A, Chaudhry I, Reid MB, Boriek AM (2002) Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem 277:46493–46503. https://doi.org/10.1074/jbc.M203654200
Ladage D, Schwinger RHG, Brixius K (2013) Cardio-selective beta-blocker: pharmacological evidence and their influence on exercise capacity. Cardiovasc Ther 31:76–83. https://doi.org/10.1111/j.1755-5922.2011.00306.x
Lau E et al (2016) A large dataset of protein dynamics in the mammalian heart proteome. Sci Data 3:160015. https://doi.org/10.1038/sdata.2016.15
Lemmon MA (2008) Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9:99–111. https://doi.org/10.1038/nrm2328
LeWinter MM, Granzier H (2010) Cardiac titin: a multifunctional giant. Circulation 121:2137–2145. https://doi.org/10.1161/circulationaha.109.860171
Li J, Russell B (2013) Phosphatidylinositol 4,5-bisphosphate regulates CapZβ1 and actin dynamics in response to mechanical strain. Am J Physiol Heart Circ Physiol 305:H1614–H1623. https://doi.org/10.1152/ajpheart.00477.2013
Li A, Ponten F, dos Remedios CG (2012) The interactome of LIM domain proteins: the contributions of LIM domain proteins to heart failure and heart development. Proteomics 12:203–225. https://doi.org/10.1002/pmic.201100492
Li J, Tanhehco EJ, Russell B (2014) Actin dynamics is rapidly regulated by the PTEN and PIP2 signaling pathways leading to myocyte hypertrophy. Am J Physiol Heart Circ Physiol 307:H1618–H1625. https://doi.org/10.1152/ajpheart.00393.2014
Lin YH, Li J, Swanson ER, Russell B (2013) CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends. J Appl Physiol (Bethesda, Md: 1985) 114:1603–1609. https://doi.org/10.1152/japplphysiol.01283.2012
Lin YH, Warren CM, Li J, McKinsey TA, Russell B (2016) Myofibril growth during cardiac hypertrophy is regulated through dual phosphorylation and acetylation of the actin capping protein CapZ. Cell Signal 28:1015–1024. https://doi.org/10.1016/j.cellsig.2016.05.011
Lin YH et al (2020) Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J Mol Cell Cardiol 139:135–147. https://doi.org/10.1016/j.yjmcc.2020.01.007
Linari M et al (2015) Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 528:276–279. https://doi.org/10.1038/nature15727
Lundby A et al (2012) Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep 2:419–431. https://doi.org/10.1016/j.celrep.2012.07.006
Ma W, Gong H, Irving T (2018) Myosin head configurations in resting and contracting murine skeletal muscle. Int J Mol Sci. https://doi.org/10.3390/ijms19092643
Martin-Garrido A et al (2018) Monophosphorylation of cardiac troponin-I at Ser-23/24 is sufficient to regulate cardiac myofibrillar Ca(2+) sensitivity and calpain-induced proteolysis. J Biol Chem 293:8588–8599. https://doi.org/10.1074/jbc.RA117.001292
McDonough JL, Arrell DK, Van Eyk JE (1999) Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res 84:9–20. https://doi.org/10.1161/01.res.84.1.9
McKenna N, Meigs JB, Wang YL (1985) Identical distribution of fluorescently labeled brain and muscle actins in living cardiac fibroblasts and myocytes. J Cell Biol 100:292–296. https://doi.org/10.1083/jcb.100.1.292
McKinsey TA (2012) Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 52:303–319. https://doi.org/10.1146/annurev-pharmtox-010611-134712
McNally EM, Amaral AP (2019) Predicting arrhythmia risk in dilated cardiomyopathy using genetic mutation status. J Am Coll Cardiol 74:1491–1493. https://doi.org/10.1016/j.jacc.2019.07.034
Mkrtschjan MA, Solis C, Wondmagegn AY, Majithia J, Russell B (2018) PKC epsilon signaling effect on actin assembly is diminished in cardiomyocytes when challenged to additional work in a stiff microenvironment. Cytoskeleton (Hoboken, NJ). https://doi.org/10.1002/cm.21472
Mohapatra B et al (2003) Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol Genet Metab 80:207–215. https://doi.org/10.1016/s1096-7192(03)00142-2
Moss RL, Fitzsimons DP, Ralphe JC (2015) Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium. Circ Res 116:183–192. https://doi.org/10.1161/circresaha.116.300561
Murphy AM, Kögler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marbán E (2000) Transgenic mouse model of stunned myocardium. Science 287:488–491. https://doi.org/10.1126/science.287.5452.488
Myburgh KH, Franks-Skiba K, Cooke R (1995) Nucleotide turnover rate measured in fully relaxed rabbit skeletal muscle myofibrils. J Gen Physiol 106:957–973. https://doi.org/10.1085/jgp.106.5.957
Nachmias VT, Golla R, Casella JF, Barron-Casella E (1996) Cap Z, a calcium insensitive capping protein in resting and activated platelets. FEBS Lett 378:258–262. https://doi.org/10.1016/0014-5793(95)01474-8
Newton AC, Antal CE, Steinberg SF (2016) Protein kinase C mechanisms that contribute to cardiac remodelling. Clin Sci 130:1499–1510. https://doi.org/10.1042/cs20160036
Nishikawa K, Lindstedt SL, Hessel A, Mishra D (2020) N2A Titin: signaling hub and mechanical switch in skeletal muscle. Int J Mol Sci 21:3974. https://doi.org/10.3390/ijms21113974
Nogara L, Naber N, Pate E, Canton M, Reggiani C, Cooke R (2016) Spectroscopic studies of the super relaxed state of skeletal muscle. PLoS ONE 11:e0160100. https://doi.org/10.1371/journal.pone.0160100
Oda T, Yanagisawa H (2020) Cryo-electron tomography of cardiac myofibrils reveals a 3D lattice spring within the Z-discs. Commun Biol 3:585. https://doi.org/10.1038/s42003-020-01321-5
Otey CA, Rachlin A, Moza M, Arneman D, Carpen O (2005) The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol 246:31–58. https://doi.org/10.1016/s0074-7696(05)46002-7
Papa I et al (1999) Alpha actinin-CapZ, an anchoring complex for thin filaments in Z-line. J Muscle Res Cell Motil 20:187–197. https://doi.org/10.1023/a:1005489319058
Perera S, Holt MR, Mankoo BS, Gautel M (2011) Developmental regulation of MURF ubiquitin ligases and autophagy proteins nbr1, p62/SQSTM1 and LC3 during cardiac myofibril assembly and turnover. Dev Biol 351:46–61. https://doi.org/10.1016/j.ydbio.2010.12.024
Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R (1997) Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res 81:404–414. https://doi.org/10.1161/01.res.81.3.404
Pinotsis N, Petoukhov M, Lange S, Svergun D, Zou P, Gautel M, Wilmanns M (2006) Evidence for a dimeric assembly of two titin/telethonin complexes induced by the telethonin C-terminus. J Struct Biol 155:239–250. https://doi.org/10.1016/j.jsb.2006.03.028
Polge C et al (2018) A muscle-specific MuRF1-E2 network requires stabilization of MuRF1-E2 complexes by telethonin, a newly identified substrate. J Cachexia, Sarcopenia Muscle 9:129–145. https://doi.org/10.1002/jcsm.12249
Potter JD, Gergely J (1974) Troponin, tripomyosin, and actin interactions in the Ca2+ion regulation of muscle contraction. Biochemistry 13:2697–2703. https://doi.org/10.1021/bi00710a007
Prill K, Dawson JF (2020) Assembly and maintenance of sarcomere thin filaments and associated diseases. Int J Mol Sci. https://doi.org/10.3390/ijms21020542
Pyle WG, La Rotta G, de Tombe PP, Sumandea MP, Solaro RJ (2006) Control of cardiac myofilament activation and PKC-βII signaling through the actin capping protein, CapZ. J Mol Cell Cardiol 41:537–543. https://doi.org/10.1016/j.yjmcc.2006.06.006
Reconditi M et al (2017) Myosin filament activation in the heart is tuned to the mechanical task. Proc Natl Acad Sci USA 114:3240–3245. https://doi.org/10.1073/pnas.1619484114
Ribeiro EA de Jr et al (2014) The structure and regulation of human muscle alpha-actinin. Cell 159:1447–1460. https://doi.org/10.1016/j.cell.2014.10.056
Risi C, Eisner J, Belknap B, Heeley DH, White HD, Schroder GF, Galkin VE (2017) Ca(2+)-induced movement of tropomyosin on native cardiac thin filaments revealed by cryoelectron microscopy. Proc Natl Acad Sci USA 114:6782–6787. https://doi.org/10.1073/pnas.1700868114
Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ (1982) The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem 257:260–263
Robia SL, Ghanta J, Robu VG, Walker JW (2001) Localization and kinetics of protein kinase C-epsilon anchoring in cardiac myocytes. Biophys J 80:2140–2151. https://doi.org/10.1016/s0006-3495(01)76187-5
Rudolph F et al (2019) Resolving titin’s lifecycle and the spatial organization of protein turnover in mouse cardiomyocytes. Proc Natl Acad Sci USA 116:25126–25136. https://doi.org/10.1073/pnas.1904385116
Rudolph F et al (2020) Deconstructing sarcomeric structure-function relations in titin-BioID knock-in mice. Nat Commun 11:3133. https://doi.org/10.1038/s41467-020-16929-8
Russell B, Motlagh D, Ashley WW (2000) Form follows function: how muscle shape is regulated by work. J Appl Physiol (Bethesda, Md: 1985) 88:1127–1132. https://doi.org/10.1152/jappl.2000.88.3.1127
Russell B, Curtis MW, Koshman YE, Samarel AM (2010) Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J Mol Cell Cardiol 48:817–823. https://doi.org/10.1016/j.yjmcc.2010.02.016
Ryder DJ, Judge SM, Beharry AW, Farnsworth CL, Silva JC, Judge AR (2015) Identification of the acetylation and ubiquitin-modified proteome during the progression of skeletal muscle atrophy. PLoS ONE 10:e0136247. https://doi.org/10.1371/journal.pone.0136247
Saad NS, Elnakish MT, Ahmed AAE, Janssen PML (2018) Protein kinase A as a promising target for heart failure drug development. Arch Med Res 49:530–537. https://doi.org/10.1016/j.arcmed.2018.12.008
Sakthivel S et al (2005) In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem 280:703–714. https://doi.org/10.1074/jbc.M409513200
Samant SA, Pillai VB, Sundaresan NR, Shroff SG, Gupta MP (2015) Histone deacetylase 3 (HDAC3)-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem 290:15559–15569. https://doi.org/10.1074/jbc.M115.653048
Samarel AM (2005) Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol 289:H2291-2301. https://doi.org/10.1152/ajpheart.00749.2005
Samarel AM, Koshman Y, Swanson ER, Russell B (2013) Biophysical forces modulate the costamere and Z-disc for sarcomere remodeling in heart failure. In: Solaro RJ, Tardiff JC (eds) Biophysics of the failing heart: physics and biology of heart muscle. Springer, New York NY, pp 141–174. https://doi.org/10.1007/978-1-4614-7678-8_7
Schafer DA, Jennings PB, Cooper JA (1996) Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol 135:169–179. https://doi.org/10.1083/jcb.135.1.169
Schmidt W, Madan A, Foster DB, Cammarato A (2020) Lysine acetylation of F-actin decreases tropomyosin-based inhibition of actomyosin activity. J Biol Chem 295:15527–15539. https://doi.org/10.1074/jbc.RA120.015277
Schramm M, Klieber HG, Daut J (1994) The energy expenditure of actomyosin-ATPase, Ca(2+)-ATPase and Na+, K(+)-ATPase in guinea-pig cardiac ventricular muscle. J Physiol 481(Pt 3):647–662. https://doi.org/10.1113/jphysiol.1994.sp020471
Scruggs SB, Solaro RJ (2011) The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys 510:129–134. https://doi.org/10.1016/j.abb.2011.02.013
Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, Buttrick PM (2010) A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain. Mol Cell Proteomics MCP 9:1804–1818. https://doi.org/10.1074/mcp.M110.000075
Scruggs SB, Wang D, Ping P (2016) PRKCE gene encoding protein kinase C-epsilon—Dual roles at sarcomeres and mitochondria in cardiomyocytes. Gene 590:90–96. https://doi.org/10.1016/j.gene.2016.06.016
Seguchi O et al (2007) A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. J Clin Investig 117:2812–2824. https://doi.org/10.1172/jci30804
Sharp WW, Simpson DG, Borg TK, Samarel AM, Terracio L (1997) Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol 273:H546-556. https://doi.org/10.1152/ajpheart.1997.273.2.H546
Sit B, Gutmann D, Iskratsch T (2019) Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing. J Muscle Res Cell Motil 40:197–209. https://doi.org/10.1007/s10974-019-09529-7
Sjöblom B, Salmazo A, Djinović-Carugo K (2008) α-Actinin structure and regulation. Cell Mol Life Sci 65:2688. https://doi.org/10.1007/s00018-008-8080-8
Smith J, Diez G, Klemm AH, Schewkunow V, Goldmann WH (2006) CapZ-lipid membrane interactions: a computer analysis. Theor Biol Med Model 3:30. https://doi.org/10.1186/1742-4682-3-30
Solaro RJ, Henze M, Kobayashi T (2013) Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res 112:355–366. https://doi.org/10.1161/circresaha.112.268672
Solís C, Russell B (2019) CapZ integrates several signaling pathways in response to mechanical stiffness. J Gen Physiol 151:660–669. https://doi.org/10.1085/jgp.201812199
Solis C, Kim GH, Moutsoglou ME, Robinson JM (2018) Ca(2+) and myosin cycle states work as allosteric effectors of troponin activation. Biophys J 115:1762–1769. https://doi.org/10.1016/j.bpj.2018.08.033
Stewart MA, Franks-Skiba K, Chen S, Cooke R (2010) Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA 107:430–435. https://doi.org/10.1073/pnas.0909468107
Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WK (2013) Posttranslational modifications of cardiac troponin T: an overview. J Mol Cell Cardiol 63:47–56. https://doi.org/10.1016/j.yjmcc.2013.07.004
Sweeney HL, Yang Z, Zhi G, Stull JT, Trybus KM (1994) Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci USA 91:1490–1494. https://doi.org/10.1073/pnas.91.4.1490
Szikora S et al (2020) Nanoscopy reveals the layered organization of the sarcomeric H-zone and I-band complexes. J Cell Biol. https://doi.org/10.1083/jcb.201907026
Tardiff JC (2011) Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res 108:765–782. https://doi.org/10.1161/circresaha.110.224170
van der Velden J, Ho CY, Tardiff JC, Olivotto I, Knollmann BC, Carrier L (2015) Research priorities in sarcomeric cardiomyopathies. Cardiovasc Res 105:449–456. https://doi.org/10.1093/cvr/cvv019
van der Ven PF et al (2000) Indications for a novel muscular dystrophy pathway. gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin. J Cell Biol 151:235–248. https://doi.org/10.1083/jcb.151.2.235
Visser MB, Koh A, Glogauer M, Ellen RP (2011) Treponema denticola major outer sheath protein induces actin assembly at free barbed ends by a PIP2-dependent uncapping mechanism in fibroblasts. PLoS ONE 6:e23736. https://doi.org/10.1371/journal.pone.0023736
Viswanathan MC, Blice-Baum AC, Schmidt W, Foster DB, Cammarato A (2015) Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance. Fronti Physiol. https://doi.org/10.3389/fphys.2015.00116
Vitale G et al (2019) The relation between sarcomere energetics and the rate of isometric tension relaxation in healthy and diseased cardiac muscle. J Muscle Res Cell Motil. https://doi.org/10.1007/s10974-019-09566-2
Wang Z et al (2010) Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92:1369–1377. https://doi.org/10.3945/ajcn.2010.29885
Ward M, Iskratsch T (2020) Mix and (mis-)match—the mechanosensing machinery in the changing environment of the developing, healthy adult and diseased heart. Biochim Biophys Acta 1867:118436. https://doi.org/10.1016/j.bbamcr.2019.01.017
Wear MA, Yamashita A, Kim K, Maéda Y, Cooper JA (2003) How capping protein binds the barbed end of the actin filament. Curr Biol CB 13:1531–1537. https://doi.org/10.1016/s0960-9822(03)00559-1
Wijnker PJ et al (2015) A novel phosphorylation site, Serine 199, in the C-terminus of cardiac troponin I regulates calcium sensitivity and susceptibility to calpain-induced proteolysis. J Mol Cell Cardiol 82:93–103. https://doi.org/10.1016/j.yjmcc.2015.03.006
Willis MS, Schisler JC, Portbury AL, Patterson C (2009) Build it up-Tear it down: protein quality control in the cardiac sarcomere. Cardiovasc Res 81:439–448. https://doi.org/10.1093/cvr/cvn289
Witt SH, Granzier H, Witt CC, Labeit S (2005) MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 350:713–722. https://doi.org/10.1016/j.jmb.2005.05.021
Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padrón R (2005) Atomic model of a myosin filament in the relaxed state. Nature 436:1195–1199. https://doi.org/10.1038/nature03920
Yamada Y, Namba K, Fujii T (2020) Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat Commun 11:153. https://doi.org/10.1038/s41467-019-14008-1
Yamashita A, Maeda K, Maéda Y (2003) Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J 22:1529–1538. https://doi.org/10.1093/emboj/cdg167
Yang Y et al (2007) Rigor-like structures from muscle myosins reveal key mechanical elements in the transduction pathways of this allosteric motor. Structure (London, England: 1993) 15:553–564. https://doi.org/10.1016/j.str.2007.03.010
Yotti R, Seidman CE, Seidman JG (2019) Advances in the genetic basis and pathogenesis of sarcomere cardiomyopathies. Annu Rev Genomics Hum Genet 20:129–153. https://doi.org/10.1146/annurev-genom-083118-015306
Young P, Gautel M (2000) The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism. EMBO Js 19:6331–6340. https://doi.org/10.1093/emboj/19.23.6331
Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM (2012) Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation 126:1828–1837. https://doi.org/10.1161/circulationaha.112.096388
Zhang D et al (2018) Converse role of class I and class IIa HDACs in the progression of atrial fibrillation. J Mol Cell Cardiol 125:39–49. https://doi.org/10.1016/j.yjmcc.2018.09.010
Zieseniss A, Terasaki AG, Gregorio CC (2008) Lasp-2 expression, localization, and ligand interactions: a new Z-disc scaffolding protein. Cell Motil Cytoskelet 65:59–72. https://doi.org/10.1002/cm.20244
Zoghbi ME, Woodhead JL, Moss RL, Craig R (2008) Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci USA 105:2386–2390. https://doi.org/10.1073/pnas.0708912105
Zou P et al (2006) Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature 439:229–233. https://doi.org/10.1038/nature04343
Acknowledgements
This work was supported by the National Institutes of Health grants HL62426 (to B. Russell, Project 2) and HL151825 (to C. Solís).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Russell, B., Solís, C. Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage. J Muscle Res Cell Motil 42, 367–380 (2021). https://doi.org/10.1007/s10974-021-09596-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-021-09596-9