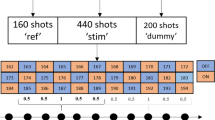
Abstract
Objective
To measure N-acetyl aspartyl glutamate (NAAG) and N-acetyl aspartate (NAA) concentrations in visual cortex activated by a continuous stimulation in a 3 T field.
Methods
NAAG and NAA spectra were obtained with MEGA-PRESS pulse sequence (TE/TR = 140/2000 ms; δONNAAG/δOFFNAAG = 4.61/4.15 ppm; δONNAA/δOFFNAA = 4.84/4.38 ppm) in 14 healthy volunteers at rest and upon stimulation by a radial checkerboard flickering at a frequency of 8 Hz. Spectra of all subjects were frequency and phase aligned and then averaged. Additionally, to obtain the time-dependency data, spectra were divided into time sections of 64 s each. The intensities of NAA, NAAG and lactate + macromolecular (Lac + MM) signals were defined by integration of the real part of spectra. The heights of the central resonance of NAAG and NAA signals were measured.
Results
The NAAG and NAA concentrations, measured with 2.5% and 0.5% error, respectively, were unaffected by visual activation. A significant increase in the Lac + MM signal by ~ 12% is clearly observed. No stimulation-induced time dependency was found for NAAG or NAA, while the increase in Lac + MM was gradual. The concentration values in visual cortex are in good agreement with the 7 T MRS measurements: [NAAG] = 1.55 mM, [NAA] = 11.95 mM.
Conclusion
The MEGA-PRESS pulse sequence together with the spectral preprocessing techniques allowed to demonstrate that the concentrations of NAAG and NAA in the visual cortex remain constant during continuous visual stimulation within the margin of error. An increase in the lactate signal intensity signifies the activation of the anaerobic glycolysis in activated visual cortex.







Similar content being viewed by others
References
Bednařík P, Tkáč I, Giove F, Dinuzzo M, Deelchand DK, Emir UE, Eberly LE, Mangia S (2015) Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab 35:601–610
Choi C, Ghose S, Uh J, Patel A, Dimitrov IE, Lu H, Douglas D, Ganji S (2010) Measurement of N-acetylaspartylglutamate in the human frontal brain by 1H-MRS at 7 T. Magn Reson Med. https://doi.org/10.1002/mrm.22536
Schaller B, Mekle R, Xin L, Kunz N, Gruetter R (2013) Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J Neurosci Res 91:1076–1083
Baslow MH, Guilfoyle DN (2016) Evidence that N-acetyaspartylglutamate is the astrocyte-targeted neurovascular coupling agent that regulates slow tonic control of brain blood flow. J Glycomics Metab. https://doi.org/10.14302/issn.2572-5424.jgm-16-1028
Guo H, Liu J, Van Shura K, Chen HZ, Flora MN, Myers TM, McDonough JH, McCabe JT (2015) N-acetyl-aspartyl-glutamate and inhibition of glutamate carboxypeptidases protects against soman-induced neuropathology. Neurotoxicology. https://doi.org/10.1016/j.neuro.2015.03.010
Neale JH, Bzdega T, Wroblewska B (2000) N-acetylaspartylglutamate: The most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. https://doi.org/10.1046/j.1471-4159.2000.0750443.x
Cangro CB, Namboodiri MAA, Sklar LA, Corigliano-Murphy A, Neale JH (1987) Immunohistochemistry and biosynthesis of N-acetylaspartylglutamate in spinal sensory ganglia. J Neurochem. https://doi.org/10.1111/j.1471-4159.1987.tb01030.x
Menshchikov PE, Semenova NA, Manzhurtsev AV, Akhadov TA, Varfolomeev SD (2018) Cerebral quantification of N-acetyl aspartate, aspartate, and glutamate levels in local structures of the human brain using J-editing of 1H magnetic resonance spectra in vivo. Russ Chem Bull 67:655–662
Mullins PG (2018) Towards a theory of functional magnetic resonance spectroscopy (fMRS): a meta-analysis and discussion of using MRS to measure changes in neurotransmitters in real time. Scand J Psychol 59:91–103
Yakovlev A, Manzhurtsev A, Menshchikov P, Ublinskiy M, Bozhko O, Akhadov T, Semenova N (2020) The effect of visual stimulation on GABA and macromolecule levels in the human brain in vivo. Biophys Russ Fed 65:51–57
Chen C, Sigurdsson HP, Pépés SE, Auer DP, Morris PG, Morgan PS, Gowland PA, Jackson SR (2017) Activation induced changes in GABA: Functional MRS at 7 T with MEGA-sLASER. Neuroimage 156:207–213
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81:89–131
McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U (2006) Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol 71:399–407
Manzhurtsev AV, Semenova NA, Ublinskii MV, Akhadov TA, Varfolomeev SD (2016) The effect of neurostimulation on the intracellular concentrations of proton-containing metabolites and macroergic phosphates in the brain cortex upon schizophrenia according to the data from 1H and 31P magnetic resonance spectroscopy. Russ Chem Bull 65:1630–1636
Mangia S, Tkáč I, Gruetter R, Van De Moortele PF, Maraviglia B, Uǧurbil K (2007) Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab 27:1055–1063
Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG (2012) Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab 32:1484–1495
Bednařík P, Tkáč I, Giove F, Eberly LE, Deelchand DK, Barreto FR, Mangia S (2018) Neurochemical responses to chromatic and achromatic stimuli in the human visual cortex. J Cereb Blood Flow Metab 38:347–359
Landim RCG, Edden RAE, Foerster B, Li LM, Covolan RJM, Castellano G (2016) Investigation of NAA and NAAG dynamics underlying visual stimulation using MEGA-PRESS in a functional MRS experiment. Magn Reson Imaging 34:239–245
Pouwels PJW, Frahm J (1997) Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed. https://doi.org/10.1002/(SICI)1099-1492(199704)10:2%3c73::AID-NBM448%3e3.0.CO;2-4
Edden RAE, Pomper MG, Barker PB (2007) In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med 57:977–982
Choi C, Ghose S, Uh J, Patel A, Dimitrov IE, Lu H, Douglas D, Ganji S (2010) Measurement of N-acetylaspartylglutamate in the human frontal brain by 1H-MRS at 7 T. Magn Reson Med 64:1247–1251
Mescher M, Tannus A, O’Neil Johnson M, Garwood M (1996) Solvent suppression using selective echo dephasing. J Magn Reson Ser A 123:226–229
Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J (2017) Advanced processing and simulation of MRS data using the FID appliance (FID-A)—an open source, MATLAB-based toolkit. Magn Reson Med 77:23–33
Govindaraju V, Young K, Maudsley AA (2000) Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 13:129–153
Vanhamme L, Van Den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Ernst T, Kreis R, Ross BD (1993) Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson Ser B 102:1–8
Penny W, Friston K, Ashburner J, Kiebel S, Nichols T (2007) Statistical parametric mapping: the analysis of functional brain images. Stat Parametr Mapp Anal Funct Brain Images. https://doi.org/10.1016/B978-0-12-372560-8.X5000-1
Alger JR (2010) Quantitative HMRS and spetroscopic imaging of the brain: a didactic review. Top Magn Reson Imaging 21:115–128
Ganji SK, Banerjee A, Patel AM, Zhao YD, Dimitrov IE, Browning JD, Sherwood Brown E, Maher EA, Choi C (2012) T 2 measurement of J-coupled metabolites in the human brain at 3T. NMR Biomed 25:523–529
Baslow MH, Hrabe J, Guilfoyle DN (2007) Dynamic relationship between neurostimulation and N-acetylaspartate metabolism in the human visual cortex: evidence that NAA functions as a molecular water pump during visual stimulation. J Mol Neurosci 32:235–245
Pouwels PJW, Frahm J (1997) Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed 10:73–78
Zhou J, Neale JH, Pomper MG, Kozikowski AP (2005) NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. https://doi.org/10.1038/nrd1903
Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T (2005) The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. https://doi.org/10.1016/j.tips.2005.07.004
Neale JH (2011) N-Acetylaspartylglutamate is an agonist at mGluR3 in vivo and in vitro. J Neurochem. https://doi.org/10.1111/j.1471-4159.2011.07380.x
Lyeth BG (2015) Application of novel therapeutic agents for CNS injury: NAAG peptidase inhibitors. Brain Neurotrauma Mol Neuropsychol Rehabil Asp. https://doi.org/10.1201/b18126
Baslow MH, Guilfoyle DN (2016) Evidence that N-acetyaspartylglutamate is the astrocyte-targeted neurovascular coupling agent that regulates slow tonic control of brain blood flow. J Glycomics Metab 1:25–36
Guo H, Liu J, Van Shura K, Chen HZ, Flora MN, Myers TM, McDonough JH, McCabe JT (2015) N-acetyl-aspartyl-glutamate and inhibition of glutamate carboxypeptidases protects against soman-induced neuropathology. Neurotoxicology 48:180–191
Forloni G, Grzanna R, Blakely RD, Coyle JT (1987) Co-localization of N-acetyl-aspartyl-glutamate in central cholinergic, noradrenergic, and serotonergic neurons. Synapse. https://doi.org/10.1002/syn.890010509
Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T (2005) The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci 26:477–484
Moffett JR, Ariyannur P, Arun P, Namboodiri AMA (2013) N-Acetylaspartate and N-acetylaspartylglutamate in central nervous system health and disease. In: Magn Reson Spectrosc Tools Neurosci Res Emerg Clin Appl Elsevier Inc., pp 71–90
Fox PT, Raichle ME, Mintun MA, Dence C (1988) Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988:80. https://doi.org/10.1126/science.3260686
Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R (1991) Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.88.13.5829
Mekle R, Kühn S, Pfeiffer H, Aydin S, Schubert F, Ittermann B (2017) Detection of metabolite changes in response to a varying visual stimulation paradigm using short-TE 1H MRS at 7 T. NMR Biomed 30:1–9
Fernandes CC, Lanz B, Chen C, Morris PG (2020) Measurement of brain lactate during visual stimulation using a long TE semi-LASER sequence at 7 T. NMR Biomed. https://doi.org/10.1002/nbm.4223
Edden RAE, Puts NAJ, Barker PB (2012) Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med 68:657–661
Chan KL, Saleh MG, Oeltzschner G, Barker PB, Edden RAE (2017) Simultaneous measurement of aspartate, NAA, and NAAG using HERMES spectral editing at 3 Tesla. Neuroimage. https://doi.org/10.1016/j.neuroimage.2017.04.043
Zhu XH, Chen W (2001) Observed BOLD effects on cerebral metabolite resonances in human visual cortex during visual stimulation: a functional 1H MRS study at 4 T. Magn Reson Med 46:841–847
Apšvalka D, Gadie A, Clemence M, Mullins PG (2015) Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. Neuroimage 118:292–300
Kauppinen RA, Pirttila TRM, Auriola SOK, Williams SR (1994) Compartmentation of cerebral glutamate in situ as detected by 1H/13C nmr. Biochem J. https://doi.org/10.1042/bj2980121
Acknowledgements
The authors of the article thank Prof. P.B. Barker and Prof. R.A.E. Edden and their team from Johns Hopkins University for the opportunity to work with the MEGA-PRESS pulse sequence. The authors would also like to thank Dr. J. Near and all the contributors for creating the FID-A toolkit for MRS data.
Funding
This study was supported by the Grants RSF 18-1300030 and RFBR 19-29-10040.
Author information
Authors and Affiliations
Contributions
AM—study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript. PM—acquisition of data, analysis and interpretation of data, critical revision. AY—analysis and interpretation of data, critical revision. MU—analysis and interpretation of data, critical revision. OB—acquisition of data, critical revision. DK—critical revision. TA—critical revision. SV—critical revision. NS—study conception and design, analysis and interpretation of data, drafting of manuscript, critical revision.
Corresponding author
Ethics declarations
Conflict of interest
Andrei Manzhurtsev declares that he has no conflict of interest. Petr Menschchikov declares that he has no conflict of interest. Alexey Yakovlev declares that he has no conflict of interest. Maxim Ublinskii declares that he has no conflict of interest. Olga Bozhko declares that she has no conflict of interest. Dmitrii Kupriyanov declares that he has no conflict of interest. Tolib Akhadov declares that he has no conflict of interest. Sergei Varfolomeev declares that he has no conflict of interest. Natalia Semenova declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manzhurtsev, A., Menschchikov, P., Yakovlev, A. et al. 3T MEGA-PRESS study of N-acetyl aspartyl glutamate and N-acetyl aspartate in activated visual cortex. Magn Reson Mater Phy 34, 555–568 (2021). https://doi.org/10.1007/s10334-021-00912-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00912-5