Abstract
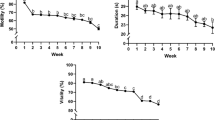
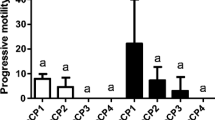
The roe deer is a monoestrous species with a very short rutting season. The present work reports the most suitable period for collecting epididymal sperm and describes the effect of two cooling rates on the post-thaw quality of sperm. Testes were collected 24–48 h after death. Samples of sperm flushed from the epididymis were subjected to either (1) dilution in a Tris-citric acid-glucose-egg yolk-based medium with glycerol, and slow freezing in straws, or (2) dilution in the same extender but replacing the glycerol with 100 mM of sucrose, and ultra-rapid freezing in pellets. Sperm motility, acrosome and membrane integrity, morphometry and morphological abnormalities were analysed before and after cryopreservation. Spermatogenic activity was investigated via histological examination of testis sections. Several testes collected between April, May and September showed no spermatogenic activity. All those collected in June–August showed spermatogenic activity. No significant difference was detected in the cryoresistance ratios associated with the conventional slow freezing, between sperm collected during the pre-rutting (April–May) and rutting (June–August) periods. No significant differences were seen between the slow-frozen-thawed and the ultra-rapid-frozen-thawed sperm in terms of percentage of viable sperm or the percentage of sperm with morphological abnormalities. Slow freezing returned significantly better (P<0.05) values for post-thaw acrosome integrity (43.3% vs. 25.0%) and straight-line velocity (19 μm/s vs. 4 μm/s). For both freezing methods, sperm heads were smaller post-thawing than pre-freezing (P<0.001). In conclusion, both the pre-rutting and rutting season are suitable periods for freezing roe deer sperm. Ultra-rapid freezing did not provide suitable results.



Similar content being viewed by others
References
Álvarez M, García-Macías V, Martínez-Pastor F, Martínez F, Borragán S, Mata M (2008) Effect of cryopreservation on head morphometry and its relation with chromatin status in brown bear (Ursus arctos) spermatozoa. Theriogenology 70:1498–1506
Álvarez-Rodríguez M, Álvarez M, Anel-López L, Guerra C, Chamorro CA, Anel L, de Paz P, Martínez-Pastor F (2018) Effect of length of time post-mortem on quality and freezing capacity of Cantabric chamois (Rupicapra pyrenaica parva) epididymal spermatozoa. Anim Reprod Sci 198:184–192
Blottner S, Hingst O, Meyer HHD (1996) Seasonal spermatogenesis and testosterone production in roe deer (Capreolus capreolus). J Reprod Fertil 108:299–305
Blottner S, Schön J, Roelant H (2007) Apoptosis is not the cause of seasonal testicular involution in roe deer. Cell Tissue Res 327:615–624
Bóveda P, Esteso MC, Castaño C, Toledano-Díaz A, López-Sebastián A, Muñiz A, Prieto P, Mejía O, Ungerfeld R, Santiago-Moreno J (2018) Slow and ultra-rapid freezing protocols for cryopreserving mouflon (Ovis musimon) and fallow deer (Dama dama) epididymal sperm. Anim Reprod Sci 192:193–199
Bóveda P, Toledano-Díaz A, Castaño C, Esteso MC, López-Sebastián A, Rizos D, Bielli A, Ungerfeld R, Santiago-Moreno J (2020) Ultra-rapid cooling of ibex sperm by spheres method does not induce a vitreous extracellular state and increases the membrane damages. PLoS One 15(1):e0227946
Braun J, Sakai M, Hochi S, Oguri N (1994) Preservation of ejaculated and epididymal stallion spermatozoa by cooling and freezing. Theriogenology 41:809–818
Coloma MA, Toledano-Díaz A, Castaño C, Velázquez R, Gómez-Brunet A, López-Sebastián A, Santiago-Moreno J (2011) Seasonal variation in reproductive physiological status in the Iberian ibex (Capra pyrenaica) and its relationship with sperm freezability. Theriogenology 76:1695–1705
Consuegra C, Crespo F, Dorado J, Ortiz I, Diaz-Jimenez M, Pereira B, Hidalgo M (2018) Comparison of different sucrose-based extenders for stallion sperm vitrification in straws. Reprod Domest Anim 53(Suppl. 2):59e61
Consuegra C, Crespo F, Dorado J, Diaz-Jimenez M, Pereira B, Ortiz I, Arenas R, Morrell JM, Hidalgo M (2019) Vitrification of large volumes of stallion sperm in comparison with spheres and conventional freezing: effect of warming procedures and sperm selection. J Equine Vet Sci 83:102680. https://doi.org/10.1016/j.jevs.2019.01.017
Cooper TG (2005) Cytoplasmic droplets: the good, the bad or just confusing? Hum Reprod 20:9–11
D’Alessandro AG, Martemucci G (2003) Evaluation of seasonal variations of semen freezability in Leccesse ram. Anim Reprod Sci 79:93–102
Dong Q, Correa LM, VandeVoort CA (2009) Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology 58:20–27
Esteso MC, Soler AJ, Fernández-Santos MR, Quintero-Moreno AA, Garde JJ (2006a) Functional significance of the sperm head morphometric size and shape for determining freezability in Iberian red deer (Cervus elaphus hispanicus) epididymal sperm samples. J Androl 27:662–670
Esteso MC, Soler AJ, Fernández-Santos MR, Quintero-Moreno AA, Garde JJ (2006b) Functional significance of the sperm head morphometric size and shape for determining freezability in Iberian red deer (Cervus elaphus hispanicus) epididymal sperm samples. J Androl 27(5):662e70
Esteso MC, Rodríguez E, Toledano-Díaz A, Castaño C, Pradiee J, López-Sebastián A (2015) Descriptive analysis of sperm head morphometry in Iberian ibex (Capra pyrenaica): optimum sampling procedure and staining methods using Sperm-Class Analyzer®. Anim Reprod Sci 155:42–49
Fernández-Santos MR, Soler AJ, Ramón M, Ros-Santaella JL, Maroto-Morales A, García-Álvarez O, Bisbal A, Garde JJ, Coloma MA, Santiago-Moreno J (2011) Effect of post-mortem time on post-thaw characteristics of Spanish ibex (Capra pyrenaica) spermatozoa. Anim Reprod Sci 129:56–66
Goeritz F, Quest M, Wagener A, Fassbender M, Broich A, Hildebrandt TB, Hofmann RR, Blottner S (2003) Seasonal timing of sperm production in roe deer: interrelationship between changes in ejaculate parameters, morphology and function of testis and accessory glands. Theriogenology 59:1487–1502
González-Fernández L, Morrell JM, Peña FJ, Macías-García B (2012) Osmotic shock induces structural damage on equine spermatozoa plasmalemma and mitochondria. Theriogenology 78:415–422
Gosch B, Fischer K (1989) Seasonal changes of testis volume and sperm quality in adult fallow deer (Dama dama) and their relationship to the antler cycle. J Reprod Fertil 85:7–17
Hidalgo M, Rodríguez I, Dorado JM (2007) The effect of cryopreservation on sperm head morphometry in Florida male goat related to sperm freezability. Anim Reprod Sci 100:61–72
Hidalgo M, Consuegra C, Dorado J, Diaz-Jimenez M, Ortiz I, Pereira B, Sanchez R, Crespo F (2018) Concentrations of non-permeable cryoprotectants and equilibration temperatures are key factors for stallion sperm vitrification success. Anim Reprod Sci 196:91e8
Holt WV (2000) Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology 53:47–58
Isachenko E, Isachenko V, Katkov I, Dessole S, Nawroth F (2003) Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod BioMed Online 6:191–200
Jimenez-Rabadan P, Garcia-Alvarez O, Vidal A, Maroto-Morales A, Iniesta-Cuerda M, Ramon M, del Olmo E, Fernández-Santos R, Garde JJ, Soler AJ (2015) Effects of vitrification on ram spermatozoa using free-egg yolk extenders. Cryobiology 71:85–90
Kozioł K, Koziorowski M (2015) Morphological defects of epididymal spermatozoa in male roe deer (Capreolus capreolus) during the reproductive season. Pol J Vet Sci 8:565–572. https://doi.org/10.1515/pjvs-2015-0073
Lincoln GA (1971) The seasonal reproductive changes in the red deer stag (CerÍus elaphus). J Zool 163:105–123
Martínez-Fresneda L, Esteso MC, Toledano-Díaz A, Castaño C, Velázquez R, López-Sebastián A, Prieto P, García-Vázquez FA, Santiago-Moreno J (2018) The percentage of egg yolk in the freezing media affects mouflon (Ovis musimon) epididymal sperm cryosurvival. Spanish. J Agric Res 16(3):e04SC04
Martínez-Fresneda L, Castaño C, Bóveda P, Tesfaye D, Schellander K, Santiago-Moreno J, García-Vázquez FA (2019a) Epididymal and ejaculated sperm differ on their response to the cryopreservation and capacitation processes in mouflon (Ovis musimon). Sci Rep 9(1):15659
Martínez-Fresneda L, O’Brien E, Velázquez R, Toledano-Díaz A, Martínez-Cáceres CM, Tesfaye D, Schellander K, García-Vázquez FA, Santiago-Moreno J (2019b) Seasonal variation in sperm freezability associated with changes in testicular germinal epithelium in domestic (Ovis aries) and wild (Ovis musimon) sheep. Reprod Fertil Dev 31:1545–1557
Martínez-Pastor F, Díaz-Corujo AR, Anel E, Herráez P, Anel L, de Paz P (2005) Post mortem time and season alter subpopulation characteristics of Iberian red deer epididymal sperm. Theriogenology 64:958–974
Martínez-Pastor F, Álvarez M, Guerra C, Chamorro CA, Anel-López L, de Paz P, Anel L, Álvarez-Rodríguez M (2019) Extender osmolality, glycerol and egg yolk on the cryopreservation of epididymal spermatozoa for gamete banking of the Cantabric chamois (Rupicapra pyrenaica parva). Theriogenology 125:109–114
McClean R, Zee YP, Holt WV, Johnston SD (2008) Cryopreservation of kangaroo spermatozoa using alternative approaches that reduce cytotoxic exposure to glycerol. Cryobiology 57:304–307
Merino O, Sanchez R, Risopatron J, Isachenko E, Katkov II, Figueroa E, Valdebenito I, Mallmann P, Isachenko V (2012) Cryoprotectant-free vitrification of fish (Oncorhynchus mykiss) spermatozoa: first report. Andrologia 44(Suppl 1):390–395
O'Brien E, Esteso MC, Castaño C, Toledano-Díaz A, Bóveda P, Martínez-Fresneda L, López-Sebastián A, Martínez-Nevado E, Guerra R, López Fernández M, Vega RS, Guillamón FG, Santiago-Moreno J (2019) Effectiveness of ultra-rapid cryopreservation of sperm from endangered species, examined by morphometric means. Theriogenology 129:160–167
Pradiee J, Esteso MC, Castaño C, Toledano-Díaz A, López-Sebastián A, Santiago-Moreno J (2014) Cryopreservation of epididymal sperm from ibexes (Capra pyrenaica) using short equilibration time with glycerol. Theriogenology 82:525–528
Pradiee J, Sánchez-Calabuig MJ, Castaño C, O`Brien E, Esteso MC, Beltrán-Breña P, Maillo V, Santiago-Moreno J, Rizos D (2018) Fertilizing capacity of vitrified epididymal sperm from Iberian ibex (Capra pyrenaica). Theriogenology 108:314–320
Prieto-Pablos MT, Sánchez-Calabuig MJ, Hildebrandt TB, Göritz F, Ortmann S, Eder S, Santiago-Moreno J, Hermes R, Saragusty J (2016) Cryopreservation of captive roe deer (Capreolus capreolus) semen. Theriogenology 86:695–703
Ramón M, Pérez-Guzman MD, Jiménez-Rabadán P, Esteso MC, García-Álvarez O, Maroto-Morales A et al (2013) Sperm cell population dynamics in ram semen during the cryopreservation process. PLoS One 8(3):e59189
Rosato MP, Iaffaldano N (2013) Cryopreservation of rabbit semen: comparing the effects of different cryoprotectants, cryoprotectant-free vitrification, and the use of albumin plus osmoprotectants on sperm survival and fertility after standard vapor freezing and vitrification. Theriogenology 79:508–516
San José C, Dorado A, Oliveros F (2007) Manual de Conservación y Gestión del Corzo Andaluz. Manuales de Conservación de la Naturaleza nº4. 93 pp. Ed. Junta de Andalucía, Consejería de Medio Ambiente, Sevilla, Spain
Sanchez R, Risopatron J, Schulz M, Villegas J, Isachenko V, Kreinberg R, Isachenko E (2011) Canine sperm vitrification with sucrose: effect on sperm function. Andrologia 43:233–241
Schön J, Göritz F, Streich J, Blottner S (2004) Histological organization of roe deer testis throughout the seasonal cycle: variable and constant components of tubular and interstitial compartment. Anat Embryol 208:151–159
Sempéré AJ, Boissin J, Dutourne B, Lacroix A, Blanc MR, Ravault JP (1983) Variations de la concentration plasmatique en prolactine, LH, et FSH et de l’activité testiculaire au cours de la première année de vie chez le Chevreuil (Capreolus capreolus L). Gen Comp Endocrinol 52:241–254
Sempéré, A.J., Mauget, R., Lacroix, A., 1992. Seasonal regulation of sexual cycle and antler growth, evidence for an endogenous rhythm. 2nd International Symposium of the Biology of Deer (ed. R.D. Brown), Springer-Verlag, New York, pp. 499-504.
Sempéré AJ, Mauget R, Mauget C (1998) Reproductive physiology of roe deer. In: Andersen R, Duncan P, Linnel JDC (eds) The European roe deer: the biology of success. Scandinavian University Press, Oslo, pp 161–188
Soler AJ, Pérez-Guzmán MD, Garde JJ (2003a) Storage of red deer epididymides for four days at 5°C: effects on sperm motility, viability, and morphological integrity. J Exp Zool 295A:188–199
Soler AJ, García AJ, Fernández-Santos MR, Esteso MC, Garde JJ (2003b) Effects of thawing procedure on postthawed in vitro viability and in vivo fertility of red deer epididymal sperm cryopreserved at -196 °C. J Androl 24:746–756
Soler AJ, Esteso MC, Fernández-Santos MR, Garde JJ (2005) Characteristics of Iberian red deer (Cervus elaphus hispanicus) spermatozoa cryopreserved after storage at 5 degrees C in the epididymis for several days. Theriogenology 64:1503–1517
Swanson WF, Bateman HL, Vansandt LM (2017) Urethral catheterization and sperm vitrification for simplified semen banking in felids. Reprod Domest Anim 52(Suppl 2):255–260
Acknowledgements
We thank the following hunting reserves for providing biological samples: Coto Regional de Caza de Morcín, Coto Regional de Caza Cabranes, Coto Regional de Caza del Cordal de Peon, Coto Regional de Caza de Valdedios, Coto Regional de Caza Sierra de Pulide, Coto Regional de Caza de Nalón, Reserva Regional de Caza de Caso, Reserva Regional de Caza de Aller, and Reserva Regional de Caza de Somiedo.
Funding
This research was funded by MINECO/AEI/FEDER and EU grant AGL2017-85753-R. P. Bóveda was the recipient of a grant for pre-doctoral researchers from MINECO (AEI/FSE, UE). Octavio Mejía was the recipient of a research fellowship from the PASPA-DGAPA-UNAM (México). V.N. Flores-Gil was funded by FONDECYT-CONCYTEC (grant contract number 000245-2015-FONDECYT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santiago-Moreno, J., Castaño, C., Bóveda, P. et al. Slow and ultra-rapid freezing protocols for cryopreserving roe deer (Capreolus capreolus) epididymal sperm collected at different times of year. Eur J Wildl Res 67, 24 (2021). https://doi.org/10.1007/s10344-021-01468-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01468-4