Abstract
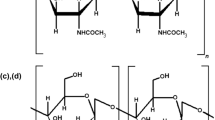
Leaf-cutting ants (genera Atta and Acromyrmex) are defoliation pests of great agronomic importance. Currently, the most effective control method is chemical using granulated baits containing sulfluramid as a standard active ingredient. In this work, a new protocol was proposed for the synthesis of the encapsulates with chitosan, tapioca, citrus pulp and sulfluramid. A sulfluramid is a pesticide which is used extensively in Brazil for management of leaf cutting ants. The main focus of this work is to use a polymeric combination aligned with the active ingredient that allows the formation of an encapsulated which the ants can transport and incorporate to their nests, being an effective methodology for the death of colonies. The encapsulated makes the active ingredient less available to the environment, but maintains the mortality level similar to the used granulated baits. For this reason, it was proposed to develop a chitosan and tapioca encapsulated for the control of leaf-cutting ants.The presence of sulfluramid in the encapsulated was confirmed by Fourier transform infrared spectrometer (FTIR), X-ray diffraction (XRD) and scanning electron microscopy (SEM). The behavioral acts related to the transport and the incorporation of leaf disks and encapsulates were observed utilizing A. sexdens colonies. The encapsulates containing sulfluramid have presented a similar intoxication of A. sexdens workers causing mortality in the same proportion as the commercial baits. The protocol for the synthesis of the encapsulates can be utilized with other substances, as entomopathogenic and parasite microorganisms.
Graphic Abstract








Similar content being viewed by others
References
De Britto JS, Forti LC, de Oliveira MA, Zanetti R, Wilcken CF, Zanuncio JC, Loeck AE, Caldato N, Nagamoto NS, Lemes PG (2016) Use of alternatives to PFOS, its salts and PFOSF for the control of leaf-cutting ants atta and acromyrmex. Int J Res Environ Stud 3:11–92
Boaretto M, Forti L (1997) Perspectives in the control of leaf-cutting ants. SérieTécnica IPEF 11(30):31–46
Mueller UG, Ishak HD, Bruschi SM, Smith CC, Herman JJ, Solomon SE, Mikheyev AS, Rabeling C, Scott JJ, Cooper M (2017) Biogeography of mutualistic fungi cultivated by leafcutter ants. MolEcol 26(24):6921–6937
De Oliveira JL, EnVR C, Pereira AE, Nunes LE, Da Silva CC, Pasquoto T, Lima R, Smaniotto G, Polanczyk RA, Fraceto LF (2018) Geraniol encapsulated in chitosan/gum arabic nanoparticles: a promising system for pest management in sustainable agriculture. J Agric Food Chem 66(21):5325–5334
Vega-Vásquez P, Mosier NS, Irudayaraj J (2020) Nanoscale drug delivery systems: from medicine to agriculture. FrontBioengBiotechnol. https://doi.org/10.3389/fbioe.2020.00079
Silva GdOd, Takizawa FF, Pedroso RA, Franco CML, Leonel M, Sarmento SBS, Demiate IM (2006) Physicochemical characteristics of modified food starches commercialized in Brazil. Food SciTechnol 26:188–197
Liu CP, Liu SD (2009) Formulation and characterization of the microencapsulated entomopathogenic fungus metarhiziumanisopliae MA126. J Microencapsul 26(5):377–384
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—A versatile semi-synthetic polymer in biomedical applications. ProgPolymSci 36(8):981–1014
Silva AM (2016) Biodegradable starch films containing encapsulated active compounds and nanoparticles: a review
Kumar MR, Muzzarelli R, Muzzarelli C, Sashiwa H, Domb A (2004) Chitosan chemistry and pharmaceutical perspectives. Chem Rev 104(12):6017–6084
Orzari LO, Santos FA, Janegitz BC (2018) Manioc starch thin film as support of reduced graphene oxide: a novel architecture for electrochemical sensors. J ElectroanalChem 823:350–358
Marichelvam M, Jawaid M, Asim M (2019) Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 7(4):32
Denardin CC, Silva LPd (2009) Starch granules structure and its regards with physicochemical properties. Ciênc Rural 39(3):945–954
Thakur R, Pristijono P, Scarlett CJ, Bowyer M, Singh S, Vuong QV (2019) Starch-based films: major factors affecting their properties. Int J BiolMacromol 132:1079–1089
Breuninger WF, Piyachomkwan K, Sriroth K (2009) Chapter 12 tapioca/cassava starch: production and use. In: BeMiller J, Whistler R (eds) Starch, 3rd edn. Academic Press, San Diego, pp 541–568
Mauruto de Oliveira GC, de Palma PE, Kunita MH, Antigo Medeiros R, de Matos R, Francisco KR, Janegitz BC (2017) Tapioca biofilm containing nitrogen-doped titanium dioxide nanoparticles for electrochemical detection of 17-β estradiol. Electroanalysis 29(11):2638–2645
Mali S, Grossmann MVE, García MA, Martino MN, Zaritzky NE (2006) Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J Food Eng 75(4):453–460
Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: processing, properties and applications. Int J BiolMacromol 105:1358–1368. https://doi.org/10.1016/j.ijbiomac.2017.07.087
Fei Liu X, Lin Guan Y, Zhi Yang D, Li Z, De Yao K (2001) Antibacterial action of chitosan and carboxymethylated chitosan. J ApplPolymSci 79(7):1324–1335. https://doi.org/10.1002/1097-4628(20010214)79:7%3c1324::AID-APP210%3e3.0.CO;2-L
Kaiser M, Pereira S, Pohl L, Ketelhut S, Kemper B, Gorzelanny C, Galla H-J, Moerschbacher BM, Goycoolea FM (2015) Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci Rep 5(1):1–14
Panos I, Acosta N, Heras A (2008) New drug delivery systems based on chitosan. Curr Drug DiscovTechnol 5(4):333–341
Sonia T, Sharma CP (2011) Chitosan and its derivatives for drug delivery perspective. Chitosan for biomaterials I. Springer, Berlin, pp 23–53
Shapi’i R, Othman SH (2016) Effect of concentration of chitosan on the mechanical, morphological and optical properties of tapioca starch film. Int Food Res J 23:S187
Chillo S, Flores S, Mastromatteo M, Conte A, Gerschenson L, Del Nobile MA (2008) Influence of glycerol and chitosan on tapioca starch-based edible film properties. J Food Eng 88(2):159–168. https://doi.org/10.1016/j.jfoodeng.2008.02.002
Salaberria AM, Diaz RH, Labidi J, Fernandes SC (2015) Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocolloids 46:93–102
Lopes J (2004) Behavioral differentiation of species of Acromyrmex spp. (Mayr, 1865) (Hymenoptera, Formicidae) cutters of monocots and dicots. Instituto de Biociências, Universidade Estadual Paulista, Botucatu, Brasil, p 93
Wilson EO (1980) Caste and division of labor in leaf-cutter ants (Hymenoptera: formicidae: atta). BehavEcolSociobiol 7(2):157–165
da Silva CR, Silva LC, Forti LC, de Matos CAO, Travaglini RV (2015) Do attasexdensrubropilosa workers prepare leaves and bait pellets in similar ways to their symbiotic fungus? Sociobiology 62(4):484–493
Nagamoto NS, Barbieri RF, Forti LC, Cardoso SRdS, Moreira SM, Lopes JFS (2011) Attractiveness of copperleaf-based bait to leaf-cutting ants. Ciênc Rural 41(6):931–934
Nagamoto NS, Forti LC, Andrade APP, Boaretto MAC, Wilcken CF (2004) Method for the evaluation of insecticidal activity over time in Atta sexdensrubropilosa workers (Hymenoptera: formicidae). Sociobiology 44(2):413–432
Bass M, Cherrett J (1995) Fungal hyphae as a source of nutrients for the leaf-cutting ant attasexdens. PhysiolEntomol 20(1):1–6
Ayres M, Ayres Jr M, Ayres D, Santos A (2007) BioEstat—Aplicaçoes estatısticas nas áreas das ciências biológicas e médicas. Belém: Sociedade Civil Mamirauá: MCT-CNPq
Hammer Ø, Harper DA (2008) Paleontological data analysis. Wiley, Hoboken
Bandeira PN, Pessoa ODL, Trevisan MTS, Lemos TLG (2002) Secondary metabolites of protiumheptaphyllum march. Quím Nov 25(6B):1078–1080
Zimmermann MV, Turella TC, Zattera AJ, Santana R (2014) Influence of the chemical treatment of banana fiber on poly(ethylene-co-vinyl acetate) composites with and without a blowing agent. Polímeros 24(1):58–64
Zhang K, Huang J, Yu G, Zhang Q, Deng S, Wang B (2013) Destruction of perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA) by ball milling. Environ SciTechnol 47(12):6471–6477
Deeyai P, Suphantharika M, Wongsagonsup R, Dangtip S (2013) Characterization of modified tapioca starch in atmospheric argon plasma under diverse humidity by FTIR spectroscopy. Chin PhysLett 30(1):018103
Vaz R, Vieira KO, Machado CE, Ferrari JL, Schiavon MA (2015) Preparation of carbon dots and their optical characterization: an experiment of nanoscience for undergraduate course. Quím Nov 38(10):1366–1373
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. ThermochimActa 396(1–2):153–166
Silva GdOd, Takizawa FF, Pedroso RA, Franco CML, Leonel M, Sarmento SBS, Demiate IM (2006) Physicochemical characteristics of modified food starches commercialized in Brazil. Food SciTechnol 26(1):188–197
Guo W, Huo S, Feng J, Lu X (2017) Adsorption of perfluorooctanesulfonate (PFOS) on corn straw-derived biochar prepared at different pyrolytic temperatures. J Taiwan InstChemEng 78:265–271
Garau MC, Simal S, Rossello C, Femenia A (2007) Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem 104(3):1014–1024
Fowler HG, Robinson S (1979) Foraging by attasexdens (Formicidae: attini): seasonal patterns, caste and efficiency. EcolEntomol 4(3):239–247
Saverschek N, Herz H, Wagner M, Roces F (2010) Avoiding plants unsuitable for the symbiotic fungus: learning and long-term memory in leaf-cutting ants. AnimBehav 79(3):689–698
Arenas A, Roces F (2017) Avoidance of plants unsuitable for the symbiotic fungus in leaf-cutting ants: learning can take place entirely at the colony dump. PLoS ONE 12(3):e0171388
Knapp J, Howse P, Kermarrec A (1990) Factors controlling foraging patterns in the leaf-cutting ant Acromyrmexoctospinosus. In: Vander Meer RK (ed) Applied mirmecology a world perspective. Westview Press, Boulder
HoČlldobler B, Wilson E (1990) The ants. Cambridge, Mass, Belknap
Okuno M, Tsuji K, Sato H, Fujisaki K (2012) Plasticity of grooming behavior against entomopathogenic fungus Metarhiziumanisopliae in the ant Lasiusjaponicus. J Ethol 30(1):23–27
Quinlan R, Cherrett J (1979) The role of fungus in the diet of the leaf-cutting ant Atta cephalotes (L.). EcolEntomol 4(2):151–160
Andrade APPd, Forti LC, Moreira AA, Boaretto MAC, Ramos VM, Matos CAOd (2002) Behavior of attasexdensrubropilosa (Hymenoptera: formicidae) workers during the preparation of the leaf substrate for symbiont fungus culture. Sociobiology 40(2):293–306
Forti LC, Pretto DR, Nagamoto NS, Padovani CR, Camargo RS, Andrade APP (2007) Dispersal of the delayed action insecticide sulfluramid in colonies of the leaf-cutting ant attasexdensrubropilosa (Hymenoptera: formicidae). Sociobiology 50(3):1149–1164
Xin S, Xiao L, Dong X, Li X, Wang Y, Hu X, Sameen DE, Qin W, Zhu B (2020) Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for schizothoraxprenati fillet preservation. Int J BiolMacromol 164:211–221. https://doi.org/10.1016/j.ijbiomac.2020.07.082
Kamaldeen O, Ariahu C, Yusufu M (2020) Application of soy protein isolate and cassava starch based film solutions as matrix for ionic encapsulation of carrot powders. J Food SciTechnol 57(11):4171–4181
Zhang J, Tang Q, Xu X, Li N (2013) Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int J Pharm 448(1):168–174. https://doi.org/10.1016/j.ijpharm.2013.03.021
Angelo LM, França D, Faez R (2021) Biodegradation and viability of chitosan-based microencapsulated fertilizers. CarbohydrPolym 257:117635. https://doi.org/10.1016/j.carbpol.2021.117635
Acknowledgments
The authors thank CNPq (303338/2019-9), FAPESP (2017/21097-3, 2019/23177-0), and CAPES (001 and 88881.504862/2020-01) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 8996 kb)
Rights and permissions
About this article
Cite this article
Gustani, F.M., Silva, T.A., Camargo, J.R. et al. Synthesis, Attractiveness and Effectiveness of Chitosan-Tapioca Encapsulates in Atta Sexdens (Hymenoptera: Formicidae). J Polym Environ 29, 2869–2880 (2021). https://doi.org/10.1007/s10924-021-02084-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02084-8