Abstract
Innovations in water technology are needed to solve challenges of climate change, resource shortages, emerging contaminants, urbanization, sustainable development and demographic changes. In particular, conventional techniques of wastewater treatment are limited by the presence of poorly biodegradable organic matter. Alternatively, recent Fenton, Fenton-like and hybrid processes appear successful for cleaning of different types of liquid wastewaters. Here, we review the application of metallic catalyst-H2O2 systems in the heterogeneous Fenton process. Each metallic catalyst-H2O2 system has unique redox properties due to metal oxidation state. Solution pH is a major influencing factor. Catalysts made of iron and cerium form stable complexes with oxidation products and H2O2, thus resulting in reduced activities. Copper forms transitory complexes with oxidation products, but copper catalytic activity is restored during the reaction. Silver and manganese do not form complexes. The catalyst performance for degradation and mineralization decreases in the order: manganese, copper, iron, silver, cerium, yet the easiness of practical application decreases in the order: copper, manganese, iron, silver, cerium.
Similar content being viewed by others
Introduction
Over the past few decades, the massive industrialization and urbanization has triggered an enormous stress on the environment. Water being the fundamental pillar of the environment has been affected the most, and numerous organic pollutants are being detected in ground- and surface waters. Water contamination has raised an alarm for the scientific community because it has serious consequences for the humans as well as to the ecosystem (Kolpin et al. 2002). To safeguard standard quality, it is crucial to carefully manage this precious resource, especially in the face of the current challenges: climate change, population growth, urbanization and pollution. Innovations in water technology are fundamental in finding solutions to these essential issues. A key feature is the pollution of anthropogenic origin constantly introduced in the environment (Bokare and Choi 2014). Nowadays, more than 700 emerging pollutants, their metabolites and transformation products, are present in the European aquatic environment. The list of emerging compounds and chemicals is significantly large and continuously growing with the introduction of new commercial compounds, disposal of chemicals and further identification of new molecules that includes pharmaceuticals and personal care products (PPCPs), pesticides, endocrine-disrupting chemicals (EDCs), industrial chemicals, surfactants and antibiotic-resistant bacteria (Gavrilescu et al. 2015). Conventional treatment processes (sedimentation and biological treatment) are not capable of removing these micropollutants, and thus innovative technologies are required (Chan et al. 2009; Glassmeyer et al. 2005; Gogate and Pandit 2004; Kasprzyk-Hordern et al. 2008; Kim et al. 2007; Metz and Ingold 2014; Quinn et al. 2008).
Advanced oxidation processes (AOPs), have been proven effective when it comes to deal with persistent organic pollutants (Andreozzi et al. 1999; Bello and Raman 2019; Boczkaj and Fernandes 2017; Glaze and Kang 1989; Mousset and Dionysiou 2020; Rueda Márquez et al. 2018; Salimi et al. 2017; Wang and Zhuan 2020). These processes generate temporary species, fundamentally hydroxyl radicals (OH•) which attack the targeted pollutants and oxidize them (Fakhru’l-Razi et al. 2009; Ioannou et al. 2015; Shahidi et al. 2015; Tiya-Djowe et al. 2016). The key features which make these processes superior to other processes are their ability to be operated near ambient conditions, nonselective nature of OH• radicals and conversion of pollutants into nontoxic products such as CO2 and H2O (Neyens and Baeyens 2003). Advanced oxidation processes can also be integrated with existing biological processes as a pretreatment strategy for the treatment of heavily polluted wastewater streams (Oller et al. 2011).
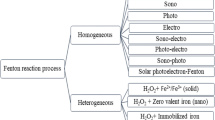
There are several types of advanced oxidation processes based on the mechanism of OH• generation: for instance, classical Fenton reaction, heterogeneous Fenton-like reaction, processes which employ any of these physical fields such as electrical, microwave, ultraviolet and ultrasonic (Comninellis et al. 2008; John and Shaike 2015; Lahkimi et al. 2007; Paramo-Vargas et al. 2016; Rayaroth et al. 2016). Advanced oxidation processes involving physical fields have not been widely adopted by the industry yet for a reliable wastewater treatment due to high energy and capital cost. Therefore, in this article we will first briefly discus Fenton reaction and finally focus on heterogeneous Fenton-like reaction (Fig. 1) because the classical Fenton reaction which is currently in place for wastewater treatment lacks processing and economic sustainability.
Homogeneous Fenton reaction
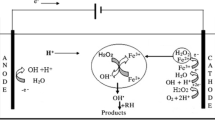
The Fenton reaction was developed by Henry John Horstman Fenton in 1890 (Barbusiński 2009; Fenton 1894). The Fenton reagent comprising of ferrous ions and an oxidant H2O2 yields transitory but extremely reactive species, i.e., hydroxyl radicals, which have remarkable oxidizing capability (Goldstein et al. 1993; Jain et al. 2018; Navalon et al. 2011; Neyens and Baeyens 2003). Although the Fenton’s reagent was discovered 100 years ago, it was not applied for the abatement of toxic organic pollutants until 1960 (Huang et al. 1993). It is critically important to comprehend the mechanism of Fenton reaction where ferrous (II) iron is mixed with H2O2, hydroxyl radicals are generated through the following chain initiation (Eq. 1) (Barhoumi et al. 2017) and chain termination (Eq. 2) reactions (Buxton et al. 1988; Rigg et al. 1954). The ferric (III) iron may also react with H2O2 and decompose it through the reaction outlined in (Eq. 3), and this particular reaction is referred as Fenton-like reaction (Walling and Goosen 1973). A series of other reactions involved in the Fenton process are outlined here (Eqs. 4–7) (Feng et al. 2013; Neyens and Baeyens 2003).
Instead of looking into all these complex reactions, Walling (Walling 1975) proposed a simplified version of Fenton reaction (Eq. 8) (Dhakshinamoorthy et al. 2012):
The \({\text{OH}}^{ \bullet }\) reacts with organics and converts them into organic radicals which undergo a series of oxidation reactions to yield secondary and tertiary metabolites (Eq. 9) (Nidheesh 2015; Nidheesh et al. 2013):
Homogeneous Fenton reaction essentially involves three processing steps: dissolution of the catalyst (de la Plata et al. 2010), OH• radical generation and finally the oxidation of organics (Fig. 2). Fenton reaction is mainly dependent on the extent of dissolution of iron catalyst, and this is the reason for which Fenton reaction does not afford good efficacy at near-neutral pH conditions. To improve the efficiency of the process, the pH of the aqueous medium has to be shifted toward acidic conditions which favors the dissolution of the catalyst. Almost all researchers have concluded that acidic conditions near pH-3 afford the best efficiency for Fenton process (Aziz and Daud 2012; Bautista et al. 2008; Deng and Englehardt 2006; Lucas and Peres 2009; Umar et al. 2010). Apart from conducive pH conditions, there are several other factors which may influence the dissolution of the catalyst and can be explained using Noyes–Whitney equation (Eq. 10) (Noyes and Whitney 1897; Otsuka et al. 2007):
where
A is surface area of the catalyst, C is concentration of the solid catalyst in the bulk dissolution medium, Cs is concentration of the solid catalyst in the diffusion layer surrounding the solid, D is diffusion coefficient, L is diffusion layer thickness.
This equation clearly suggests that catalyst surface area plays a critical role and is proportional to the rate of dissolution of the catalyst. Further, larger quantities of catalyst also enhance the solubility of solid due to higher concentration gradient between liquid and solid phases. Moreover, the characteristics of dissolving medium, i.e., wastewater, also govern the solubility of catalyst. It is also important to note that the nature of the iron catalyst may also affect the dissolution rate.
Once the catalyst is dissolved, the Fe+2 ions start producing OH• radicals from the oxidant. The rate of OH• radicals mainly depends on the concentrations of both the catalyst and the oxidant. However, excess amounts of either of these entities beyond optimal conditions may also trigger a scavenging effect which may hinder the process efficiency (Eqs. 11 and 12) (Aşçı 2013; Nasuha et al. 2016):
Therefore, to avoid the adverse effects of scavenging phenomenon, Fenton process must be optimized with respect to catalyst and oxidant doses.
The transitory OH• radicals then attack on the organic molecules and abstract one of their hydrogen atoms and turn them into R• which ultimately undergoes a series of oxidation reactions to yield secondary and tertiary products, ideally H2O and CO2. Organics must go through the oxidation process, and consequently, the nature of the organics not only affects the extent of oxidation but also the quality of the finally treated wastewater. Therefore, hydrocarbons with stable and high molecular weights tend to yield relatively stable radicals which are difficult to oxidize. Another factor which hinders their oxidation is their poor solubility in the aqueous medium because homogeneous Fenton reaction must take place in the solution phase. The order of stability and consequently the difficulty posed by organic pollutants to undergo oxidation are illustrated in Fig. 3 (Perathoner and Centi 2005).
Limitations of Fenton process
Fenton process has many advantages such as processing of wastewater at ambient conditions, high reaction rate between H2O2 & Fe (II) (Pouran et al. 2014), nontoxic reagents and convenience of integration with existing treatment facilities (Brillas et al. 2009). Moreover, Fenton process has been successfully employed for the treatment of numerous industrial wastewaters (Aziz and Daud 2012; Deng and Englehardt 2006; Lucas and Peres 2009; Mosteo et al. 2007; Soares et al. 2014; Wang et al. 2016). However, homogeneous Fenton reaction is only feasible when pH is lower than 4 because the interconversion of Fe+2 and Fe+3 maximizes the process efficiency (Tang et al. 2019). When pH exceeds 4, Fe+3 is converted into ferric hydroxide sludge and part of the catalyst is lost and hence efficacy of the Fenton reaction declines (Garrido-Ramírez et al. 2010). The rigid acidic conditions require constant addition of chemicals before and after wastewater treatment and thus lead the process toward economic nonfeasibility. Besides, handling and disposal of solid sludge incur additional costs. Moreover, the newly formed sludge may also serve as an absorbent for the pollutants in the wastewater and hence give rise to another environmental hazard. Furthermore, recycling of the iron sludge is also not feasible.
Heterogeneous Fenton-like reaction
Fenton-like reaction is established when Fe+2 is either replaced with Fe+3 or other transition metal ions in the Fenton reagent system (Wang et al. 2016). Although heterogeneous Fenton-like reaction also coexists within homogeneous Fenton reaction, it is limited because of the narrow pH range and quickly dissipates once favorable conditions are inverted (Caudo et al. 2006). Heterogeneous Fenton-like reaction can be successfully used to overcome the processing and economic constraints associated with homogeneous Fenton reaction such as high input of chemicals, catalyst loss and large amount of sludge generation (Centi et al. 2000; Navalon et al. 2010). In heterogeneous Fenton reaction, the Fe+3 is principally used in the nonsoluble form either harnessing naturally occurring minerals such as magnetite (Fe3O4), maghemite (γ-Fe2O3), hematite (α-Fe2O3) and pyrite (FeS2) (Feng et al. 2012; Pereira et al. 2012) or impregnating it over suitable supports to afford extended surface area (Flores et al. 2008; Gumy et al. 2005; Muthuvel and Swaminathan 2008; Xue et al. 2009).
Heterogeneous Fenton process is altogether different when compared with homogeneous Fenton process because adsorption is mainly responsible for determining the efficiency of the process. There are three steps involved in heterogeneous Fenton process: adsorption of organics over the catalyst surface, in situ generation and attack of OH• radicals on organics (He et al. 2016) and finally desorption of oxidation products from catalyst surface (Fig. 4). In order to explain the driving force of adsorption in heterogeneous Fenton oxidation, Langmuir equation can be used (Eq. 13):
where
Heterogeneous Fenton process. Heterogeneous Fenton reaction essentially involves three processing steps: adsorption of organics over the catalyst surface, \({\text{OH}}^{ \bullet }\) radicals generation through active sites on the catalyst surface and oxidation of the organics, desorption of the oxidation products from the catalysts surface
Ce is equilibrium concentration of organics, Qe is equilibrium monolayer adsorption capacity, Qm is complete monolayer adsorption capacity, Kl is Langmuir adsorption constant.
The equation clearly suggests that the rate of adsorption predominantly depends upon the monolayer adsorption capacity of the solid catalyst. This further suggests that higher surface area of the catalyst is exposed in the aqueous medium, resulting in a higher organic adsorption (Geçgel et al. 2015). Although multilayer adsorption may also exist, it only happens when organics are present in very high concentrations. Once the organics are adsorbed onto catalyst surface, OH• radicals are generated through active sites on the catalyst surface and start oxidizing the organics. The oxidation products either undergo further oxidation or desorb from the catalysts surface, completing the heterogeneous catalytic cycle.
Originally, heterogeneous Fenton process was developed by harnessing iron (Fe+3) to overcome the disadvantages of homogeneous Fenton process, mitigating several processing constraints such as reduced sludge generation, lower chemical input and hence lower cost. However, pH optimization and control remain the major defect in the process because in order to afford high efficiency, acidic conditions are favorable but in doing so metal ions start coming off from the catalyst surface. Leaching of the active metal from the catalyst surface inevitably results in lowering the catalytic activity, turning the process less sustainable. Therefore, many researchers have been striving to employ various metals including iron to develop heterogeneous catalysts with enhanced stability without compromising the acceptable activity threshold. Now, we will discuss frequently used metals for the development of heterogeneous catalyst for Fenton oxidation and critically analyze their performance in terms of activity and stability. Specifically, we will focus on iron, cerium, copper, manganese and silver catalysts.
Iron-based catalysts have been mainly discussed because the main idea of the Fenton and Fenton-like processes originated using iron-containing materials. This is the only metal which forms stable complexes with the degradation products.
Copper-based materials have been considered due to the redox cycle that is very similar to that of iron. Moreover, unlike iron, copper forms temporary complexes with the degradation products.
Silver has been chosen because of its unique redox cycle involving elemental Ag and Ag+.
Cerium has been taken into consideration because this is the only metal when employed in Fenton-like oxidation process forms complexes with the oxidant.
Manganese has been included in the review because it offers two separate redox cycles depending upon the nature of manganese-based material.
Role of metals in heterogeneous Fenton-like oxidation
Considerations for development of sustainable catalyst
The fundamental prerequisites to develop a sustainable heterogeneous catalyst for Fenton oxidation are high activity and stability. In order to achieve these features in a catalyst, it is essential that the metal used for catalyst development can exist in multiple oxidation states due to its higher capacity to transform H2O2 into OH• radicals (Bokare and Choi 2014). Moreover, all these oxidation states ought to be stable over a wide range of pH to avoid the loss of catalyst though leaching. Another aspect which must be considered is the resistance of the metallic species toward hydration forces/nonsoluble nature of the metallic ions. Apart from these requirements, the metal entities must have the potential to transform pollutants into terminal oxidation products, i.e., CO2 and H2O.
Iron
The abundant and cost-effective availability of iron is the prime reason due to which researchers are still focused to develop heterogeneous catalysts by employing this metal (Pereira et al. 2012). Another reason for harnessing this metal is its high activity in Fenton process and familiarity with reaction mechanism (Pouran et al. 2014). Iron-based heterogeneous catalysts are still the most widely used catalysts in Fenton-like process, and many researchers have reported their findings which are presented in Table 1. Iron can exhibit its heterogeneous catalytic features cycling between Fe3+ and Fe2+ (Feng et al. 2013; Hartmann et al. 2010; Rusevova et al. 2012); some researchers have also proposed high-valent iron species such as ferryl (Fe+4) but these species only exist in basic conditions (Gonzalez-Olmos et al. 2011; He et al. 2016; Luo et al. 2010).
Controlling parameters
The nature of the iron-based catalyst is the governing parameter which dictates rest of the factors for optimal performance of the catalyst in Fenton process because coordination of the iron species in different catalytic environments is inherently different; the nature of the catalyst predominantly regulates the optimization of the parameters in Fenton process (Wang et al. 2013). For example, there is a marked difference of optimal pH between BiFeO3 and zerovalent iron (Table 1). Likewise, similar iron-based catalysts such as nanoparticle iron and zerovalent iron yield maximum activity at identical pH conditions.
The parameters pH, catalyst dose, oxidant dose, temperature, reaction time and pollutant type will strongly affect the efficiency of the Fenton process. The most critical parameter for iron-based heterogeneous catalysis is the pH of the wastewater. Acidic pH conditions yield higher activity of the process because part of the iron species is lost into the solution phase and may contribute toward improving efficiency of the process through partial homogeneous Fenton reaction (Rusevova et al. 2014). However, this will also cause substantial metal loss from catalyst surface and turn the catalyst less active in subsequent cycles. Moreover, acidic pH conditions of the aqueous solutions are adjusted by the addition of HCl or H2SO4 and this will increase the concentration of SO42− and Cl− ions, which are known as inhibitors for the generation of OH• radicals, thus adversely affecting the overall process efficacy (Lin et al. 2015).
Another important parameters are the catalyst dose and the associated surface area of the catalyst. Generally, higher catalyst loadings favor the efficacy of the Fenton process, especially when iron-based heterogeneous catalysts are used, but beyond a certain point it may also negatively impact the efficacy due to scavenging effect, i.e., consumption of OH• radicals by the catalyst itself (Chen et al. 2015b; Divya and Renuka 2015). Heterogeneous Fenton process is a surface phenomenon and higher surface area of the catalyst will increase the effectiveness of the catalyst. However, extended surface area of iron-based catalysts may subject them to strong hydration forces, especially in acidic conditions, and ultimately result in the increase in metal loss (Duarte et al. 2012).
Obviously, oxidant dose is also critical when it comes to achieve optimal efficiency in Fenton process. However, it has been observed that when iron-based catalysts are used, only a small excess of oxidant is required to obtain maximum pollutant abatement (Chen et al. 2015b; Duarte et al. 2012). It is also worth mentioning that the type of the pollutants will regulate not only the extent of oxidation but also the degree to which oxidation objectives are achieved, i.e., whether a mere degradation of the organics is required, or a complete mineralization is the primary objective.
A key characteristic of the Fenton-like process carried out by iron-based catalysts is their ability to degrade wide range of organics (Zhou et al. 2008). Further, iron-based catalysts afford high reaction rates in terms of organic degradation, but rate of mineralization is far slower due to two possible reasons. First, the oxidation products do not desorb from catalyst surface; second, due to their inability to generate in situ R• radicals from secondary oxidation products because Fe+3 forms very stable complexes with oxidation products and ultimately inhibits the oxidation of degraded products as shown in Fig. 5 (Salazar et al. 2012; Sirés et al. 2006; Vindedahl et al. 2016).
Copper
Copper is the second most used transition metal in heterogeneous Fenton process because of several characteristics such as inexpensiveness, abundant availability, nontoxic nature and high activity. Another feature which has attracted the attention of research community is its similar redox behavior like iron. There are two oxidation states of copper, i.e., cuprous (Cu+) and cupric (Cu+2), which can react with H2O2 to form OH• radicals (Bokare and Choi 2014). However, copper has a distinct property which makes it even a better catalytic entity when compared with iron, its ability to form temporary complexes with oxidation products and rapid interconversion of Cu+ into Cu+2 and vice versa (Lyu et al. 2015). The oxidation products do not form permanent complexes with copper, and hence the active sites remain available for continuous catalytic cycle (Fig. 6). Therefore, copper not only offers better redox cycle, but is also active in the mineralization of organics. Several researchers have employed copper in variable forms as heterogeneous catalyst in Fenton process (Table 2).
Controlling parameters
The principal feature of copper-based heterogeneous catalysts is their potential to perform well over a broad pH range, especially at near-neutral pH conditions. However, the optimal pH conditions depend upon the nature of the catalyst and its corresponding value of point of zero charge. Unlike iron catalysts, acidic conditions not only reduce the overall activity of these materials but also amplify the loss of metal from catalyst surface. Moreover, copper-based catalysts do not offer catalytic activity through homogeneous phase. Another advantage of copper over iron is its ability to afford better catalytic activities with lower catalyst dose because it possesses superior redox cycle and extended catalyst stability. Further, heterogeneous catalysis carried out by copper made catalysts is much faster. However, optimal catalyst dose ought to be determined experimentally and any additional amount of catalyst will bear strong scavenging effects and ultimately process efficiency will decline.
Copper-based catalysts have a serious disadvantage concerning oxidant dose: a fairly large excess of oxidant is required to obtain optimal pollutant abatement because molecular oxygen disturbs the redox cycle of copper and part of the oxidant is lost in the process. The large excess of H2O2 not only increases the cost of the Fenton process but also makes it susceptible to severe scavenging effect caused by the oxidant itself for OH• radicals (Bali and Karagözoğlu 2007; Miles and Brezonik 1981).
Contrary to iron, the activity of the copper-based catalysts is greatly influenced by variations in the reaction temperature. Higher reaction temperature favors the rate as well as efficiency of the Fenton process because the energy required by the organics and H2O2 to form oxidation products is supplied through an elevation in temperature (Konstantinou and Albanis 2004; Nasuha et al. 2016). However, beyond a certain point, temperature may also negatively impact the process efficiency due to the formation of undesirable stable oxidation products, loss of active sites through hydration forces and decomposition of oxidant into useless species.
Cu-based catalysts have a main disadvantage which is the high excess of H2O2 requirement for maintaining the catalytic activity. This disadvantage has often been mitigated by employing a bimetallic composite of copper with iron and other transition metals. However, iron has been used more frequently and various studies have shown promising results in this regard. The induction of two metals not only enhances the catalytic activity but also increases the stability of the catalytic composite because of superior redox cycle (Fig. 7). Tian et.al. have summarized the redox mechanism of bimetallic redox cycle using a single equation (Eq. 14) (Tian et al. 2017):
Fe3+ is reduced to Fe2+ while Cu+ is oxidized to Cu2+, favoring the oxidation of organic matter.
Silver
The main drive to use silver as a catalyst in Fenton-like process is its proven ability to oxidize organics such as methanol (Kundakovic and Flytzani-Stephanopoulos 1999), ethylene (Mao and Vannice 1995), methane and volatile organic compounds (Qu et al. 2005). He et.al. reported the use of silver nanoparticles for the generation of OH• from H2O2 (He et al. 2012). Until now, silver has not been widely studied as a heterogeneous Fenton catalyst (Table 3).
Controlling parameters
In the context of Fenton-like oxidation, silver can exist in two oxidation forms, i.e., Ag0 and Ag+1, depending upon the pH conditions of the aqueous medium; therefore, pH is the most crucial parameter (Saeed et al. 2018). Under acidic conditions, bare silver will tend to dissolve in the water phase and will transform H2O2 into OH• radicals through homogeneous phase. However, basic conditions will shift the redox reaction in the opposite direction and instead of OH• radical generation, O2 is produced through heterogeneous phase reaction (Fig. 7). Keeping in view of these facts, silver can efficiently be used as heterogeneous catalyst when pH conditions are either neutral or basic. However, the unfavorable redox reactions will suppress the catalytic activity while the conditions become basic as suggested by Weaver and Frederikse (Eq. 15, and 16) (Weaver and Frederikse 1977). Additionally, basic conditions may activate the agglomeration of the catalyst particles, reducing surface area and diminishing the activity (Park et al. 2017). Moreover, with the progress of Fenton reaction, the degradation products being predominantly acidic will acidify the aqueous medium and silver will start leaching out from the solid surface:
These drawbacks associated with silver can be averted by incorporating an appropriate support material such as ceria, zirconia, etc. The integration of silver on supports with oxygen storage capacity will not only enhance its activity but also strengthen the catalytic structure because of dual redox cycle, one responsible for activity and the other for stability (Fig. 8). Moreover, surface area is also increased manifold by the introduction of supports which will ultimately increase the activity of the catalyst. Further, the catalyst can afford viable activities through broader pH range due to dual redox cycle.
The oxidant dose directly affects the silver-catalyzed heterogeneous Fenton process; however, only a small excess of stoichiometric oxidant dose is sufficient to achieve optimal efficacy. Further, a large excess of oxidant dose ought to be avoided because it can either cause scavenging effect or lower the pH and hence catalytic activity is subdued (Park et al. 2017). The most prominent feature of silver formed catalysts is that they can afford equivalent process efficacies with minimal catalyst loadings.
Cerium
Cerium is a rare earth metal from lanthanide group which has been widely employed in wet air oxidation and water gas shift reactions (Aneggi et al. 2016; Trovarelli et al. 1999). Owing to its oxidation properties, cerium can conveniently produce OH• from H2O2 due to exhibition of two oxidation states, i.e., + 3 and + 4. Heckert et al. used cerium-based heterogeneous catalysts for the production of OH• from H2O2 through the mechanism outlined in Eq. 17 and 18 (Heckert et al. 2008b). It is important to note that cerous (Ce+3) is a strong reducing agent, while ceric (Ce4+) is a strong oxidizing agent. These two ions can interchange quite easily, offering a good redox cycle which is critical for heterogeneous Fenton like oxidation (Aneggi et al. 2012; Rossi et al. 2012). A list of studies employing cerium in Fenton-like oxidation is presented in Table 4.
Controlling parameters
Unlike other metals, the favorable redox cycle of cerium in aqueous environments is very much dependent on the pH of the medium. Therefore, pH of the polluted water is the most critical parameter which governs the activity of cerium catalyst. Under basic conditions, H2O2 forms very stable peroxide-like species (OOH−) with cerium (Chen et al. 2012) and these species do not decompose even at neutral pH conditions; thus, no OH• radicals are generated at all because the redox cycle between Ce4+/ Ce3+ is completely blocked (Cai et al. 2010; Heckert et al. 2008a; Ji et al. 2010). On the contrary, when acidic conditions are available, the H+ ions attack the cerium-peroxide complex and redox cycle is unblocked which yields OH• radicals (Fig. 9). However, it is important to note that the cerium-peroxide complex will form under both conditions and will only decompose when acidic conditions are applied.
Cerium reactions with H2O2 under acidic and basic conditions. Under basic conditions, H2O2 forms very stable peroxide-like species (OOH−) with cerium inhibiting \({\text{OH}}^{ \bullet }\) radicals generation, while in acidic conditions, H+ ions attack the cerium-peroxide complex with \({\text{OH}}^{ \bullet }\) radicals generation
Apart from using an experimentally determined optimal dose of oxidant, it is also crucial to use it in a suitable processing fashion which favors the Fenton oxidation; i.e., oxidant should never be premixed with the cerium catalyst because it is highly likely that it will block the catalytic activity (Heckert et al. 2008a). Therefore, it is viable to employ the oxidant as a last processing step so that part of the cerium catalyst sites is preoccupied by the organics and partly by cerium-peroxide complexes. Further, higher oxidant dose will only intensify the blockage of catalytic activity of cerium. The cerium-peroxide complexes are reverted by acidic conditions or either by applying very high temperatures ~ 300 °C (Ferrizz et al. 2001; Liu et al. 2009). Since an increase in temperature is beneficial in reversing the cerium-peroxide complex which triggers the redox cycle Ce4+/ Ce3+, any elevation in temperature would certainly enhance the heterogeneous activity of cerium catalyst (Ferrizz et al. 2001).
Manganese
Manganese is a transition metal, which is abundantly available, has low toxicity, is not very expensive, and exists in multiple oxidation states (Ren et al. 2012). Manganese offers structural flexibility in its metallic oxides while exhibiting the favorable oxidation states (Birkner et al. 2013). However, Mn+2 and Mn+4 are the only suitable oxidation states while considering heterogeneous Fenton-like oxidation as reported in many studies (Table 5) (Robinson et al. 2013). It is important to mention that manganese transforms H2O2 into OH• radicals by undergoing through a transitional intermediate, i.e., Mn+3 (Rhadfi et al. 2010; Sigel 2000; Watts et al. 2005), thus possessing a unique redox cycles depending upon the type of oxide, and an electron is exchanged between the substrate and the solution (Fig. 10) (Parida et al. 2005).
Controlling parameters
The most critical parameter which dictates the efficacy of manganese catalyzed Fenton oxidation is the pH of the aqueous medium because the pH conditions change altogether when MnO2 and Mn3O4 are used (Fig. 10). When Mn3O4 is employed, the reaction is favored by a basic environment (Eqs. 19 and 20), though neutral conditions are applied for practical reasons:
Conversely, MnO2 requires acidic conditions to drive the redox cycle toward Mn+3 generation which in turn transforms H2O2 into OH• radicals (Eqs. 21 and 22). Additionally, precise control of the pH is also essential because adsorption of organics over the catalyst surface is influenced by it (Zhao et al. 2013). Moreover, the oxidation pathway of organics as well as stability of the catalyst heavily relies on the pH:
In the case of manganese-based catalysts, another parameter which greatly influences their performance in Fenton-like process is their morphology (Hermanek et al. 2007; Kim et al. 2017). For instance, MnO2 can exist in four crystalline structures, i.e., α- MnO2, β-MnO2, γ-MnO2, δ-MnO2. All of them have different surface areas and extents of crystallinity, thus exhibiting variable catalytic activities in heterogeneous Fenton process (Xiao et al. 2010). γ-MnO2 affords maximum activity because of well-formed morphology and high surface area, while δ-MnO2 is the least active because it is an amorphous solid with minimum surface area (Kim et al. 2017). Kim et.al. also explained that different morphologies of MnO2 have different magnetic moments, indicating that these oxides exhibit mixed oxidation states (Kim et al. 2017).
H2O2 dose has a direct impact on the efficacy of manganese-driven Fenton-like process because more oxidant is available for OH• radical generation. However, an optimal oxidant dose has always to be determined experimentally because excess dose may give rise to scavenging effect which negatively affects the process efficiency. Moreover, any excessive oxidant dose may also disturb the pH balance of the solution which may suppress the activity of the catalyst (Molina et al. 2006). Similarly, increasing the manganese catalyst loading also increases the overall efficiency of the Fenton process due to increase in the number of active sites through which OH. radicals are generated, and organics are attacked. However, beyond a certain point, surplus catalyst starts moderating the potency of the process due to scavenging effect and aggregation of the material which reduces the exposed active sites of the catalyst. In accordance with Arrhenius law, a rise in temperature elevates the activity of manganese catalyst because lower amount of activation energy is required for product formation (Xiao et al. 2010).
Conclusion
The inborn limitations of classical Fenton process such as large volumes of sludge and stringent pH prerequisites can be easily reverted by adapting heterogeneous Fenton-like approach, employing either iron or other metallic systems. The choice of an appropriate metal not only offers milder pH conditions but also greatly enhances the efficacy of the Fenton process by providing alternate and better redox cycle. In this review, we have discussed different metals and their suitability in Fenton process, considering all the processing factors (Fig. 11). Iron-based catalysts require severe acidic conditions, high catalyst doses, they form stable complexes with oxidation products, and complete mineralization of organics is difficult to achieve. However, these catalysts can bear optimum activities with minimal H2O2 excess and the energy required to produce oxidizing species is the lowest among the discussed metals. Although silver-based catalysts require less excess H2O2, low catalyst loadings for optimal performance, a poor redox cycle coupled with susceptibility toward leaching limits their application in Fenton process unless a proper support is employed. Cerium-based catalysts form very stable complexes with the oxidant and can only be broken if stringent acidic conditions are applied. Besides, they require the highest catalyst loadings and excess H2O2. Copper and manganese both possess superior redox cycles, require feasible catalyst loadings, excess H2O2 and can afford optimum activities under flexible pH conditions, and they have the ability to completely mineralize the organics.
Code availability
Not applicable.
References
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery Catalysis. Today 53:51–59. https://doi.org/10.1016/S0920-5861(99)00102-9
Aneggi E, Cabbai V, Trovarelli A, Goi D (2012) Potential of Ceria-Based Catalysts for the Oxidation of Landfill Leachate by Heterogeneous Fenton Process Artn 694721 https://doi.org/10.1155/2012/694721
Aneggi E, Boaro M, Colussi S, de Leitenburg C, Trovarelli A (2016) Ceria-Based Materials in Catalysis: Historical Perspective and Future Trends Handbook on the Physics and Chemistry of Rare Earths 50: 209-242 https://doi.org/10.1016/bs.hpcre.2016.05.002
Aneggi E, Trovarelli A, Goi D (2017) Degradation of phenol in wastewaters via heterogeneous Fenton-like Ag/CeO2 catalyst. J Environ Chem Eng 5:1159–1165. https://doi.org/10.1016/j.jece.2017.01.042
Araujo F, Yokoyama L, Teixeira L, Campos J (2011) Heterogeneous Fenton process using the mineral hematite for the discolouration of a reactive dye solution. Braz J Chem Eng 28:605–616. https://doi.org/10.1590/S0104-66322011000400006
Asci Y (2013) Decolorization of Direct Orange 26 by heterogeneous Fenton oxidation. Desalination and Water Treatment, 51: 7612–7620 https://doi.org/10.1080/19443994.2013.776504
Ayoub H et al (2018) Iron-impregnated zeolite catalyst for efficient removal of micropollutants at very low concentration from Meurthe river. Environ Sci Pollut Res 25:34950–34967. https://doi.org/10.1007/s11356-018-1214-0
Aziz AA, Daud WMAW (2012) Oxidative mineralisation of petroleum refinery effluent using Fenton-like process. Chem Eng Res Design 90:298–307. https://doi.org/10.1016/j.cherd.2011.06.010
Bali U, Karagözoğlu B (2007) Performance comparison of Fenton process, ferric coagulation and H2O2/pyridine/Cu(II) system for decolorization of Remazol Turquoise Blue G-133. Dyes pigments 74:73–80. https://doi.org/10.1016/j.dyepig.2006.01.013
Barbusiński K (2009) Henry John Horstman Fenton-short biography and brief history of Fenton reagent discovery. Chem Didact Ecol Metrol 14:101
Barhoumi N, Oturan N, Ammar S, Gadri A, Oturan MA, Brillas E (2017) Enhanced degradation of the antibiotic tetracycline by heterogeneous electro-Fenton with pyrite catalysis. Environ Chem Lett 15:689–693. https://doi.org/10.1007/s10311-017-0638-y
Barreto-Rodrigues M, Silveira J, García-Muñoz P, Rodriguez JJ (2017) Dechlorination and oxidative degradation of 4-chlorophenol with nanostructured iron-silver alginate beads. J Environ Chem Eng 5:838–842. https://doi.org/10.1016/j.jece.2016.12.051
Bautista P, Mohedano A, Casas J, Zazo J, Rodriguez J (2008) An overview of the application of Fenton oxidation to industrial wastewaters treatment. J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 83:1323–1338. https://doi.org/10.1002/jctb.1988
Bayat M, Sohrabi M, Royaee SJ (2012) Degradation of phenol by heterogeneous Fenton reaction using Fe/clinoptilolite. J Ind Eng Chem 18:957–962. https://doi.org/10.1016/j.jiec.2011.09.004
Bello MM, Raman AAA (2019) Synergy of adsorption and advanced oxidation processes in recalcitrant wastewater treatment. Environ Chem Lett 17:1125–1142. https://doi.org/10.1007/s10311-018-00842-0
Birkner N, Nayeri S, Pashaei B, Najafpour MM, Casey WH, Navrotsky A (2013) Energetic basis of catalytic activity of layered nanophase calcium manganese oxides for water oxidation. Proc Natl Acad Sci 110:8801–8806. https://doi.org/10.1073/pnas.1306623110
Bobu M, Yediler A, Siminiceanu I, Schulte-Hostede S (2008) Degradation studies of ciprofloxacin on a pillared iron catalyst. Appl Catal B 83:15–23. https://doi.org/10.1016/j.apcatb.2008.01.029
Boczkaj G, Fernandes A (2017) Wastewater treatment by means of advanced oxidation processes at basic pH conditions: a review. Chem Eng J 320:608–633. https://doi.org/10.1016/j.cej.2017.03.084
Bokare AD, Choi W (2014) Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater 275:121–135. https://doi.org/10.1016/j.jhazmat.2014.04.054
Bradu C, Frunza L, Mihalche N, Avramescu S-M, Neaţă M, Udrea I (2010) Removal of Reactive Black 5 azo dye from aqueous solutions by catalytic oxidation using CuO/Al2O3 and NiO/Al2O3. Appl Catal B: Environ 96:548–556. https://doi.org/10.1016/j.apcatb.2010.03.019
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631. https://doi.org/10.1021/cr900136g
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in aqueous solution. J Phys Chem Refer Data 17:513–886. https://doi.org/10.1063/1.555805
Cai W, Chen F, Shen X, Chen L, Zhang J (2010) Enhanced catalytic degradation of AO7 in the CeO2–H2O2 system with Fe3+ doping. Appl Catal B: Environ 101:160–168. https://doi.org/10.1016/j.apcatb.2010.09.031
Caudo S, Centi G, Genovese C, Perathoner S (2006) Homogeneous versus heterogeneous catalytic reactions to eliminate organics from waste water using H2O2. Topics Catalysis 40:207–219. https://doi.org/10.1007/s11244-006-0122-6
Centi G, Perathoner S, Torre T, Verduna MG (2000) Catalytic wet oxidation with H2O2 of carboxylic acids on homogeneous and heterogeneous Fenton-type Catalysts. Catal Today 55:61–69. https://doi.org/10.1016/S0920-5861(99)00226-6
Chan YJ, Chong MF, Law CL, Hassell D (2009) A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J 155:1–18. https://doi.org/10.1016/j.cej.2009.06.041
Chen F, Shen X, Wang Y, Zhang J (2012) CeO2/ H2O2 system catalytic oxidation mechanism study via a kinetics investigation to the degradation of acid orange 7. Appl Catal B: Environ 121:223–229. https://doi.org/10.1016/j.apcatb.2012.04.014
Chen H, Zhang L, Zeng H, Yin D, Zhai Q, Zhao X, Li J (2015a) Highly active iron-containing silicotungstate catalyst for heterogeneous Fenton oxidation of 4-chlorophenol. J Mol Catal A: Chem 406:72–77. https://doi.org/10.1016/j.cej.2015.03.079
Chen H, Zhang Z, Yang Z, Yang Q, Li B, Bai Z (2015b) Heterogeneous fenton-like catalytic degradation of 2, 4-dichlorophenoxyacetic acid in water with FeS. Chem Eng J 273:481–489. https://doi.org/10.1016/j.molcata.2015.05.017
Cihanoğlu A, Gündüz G, Dükkancı M (2015) Degradation of acetic acid by heterogeneous Fenton-like oxidation over iron-containing ZSM-5 zeolites. Appl Catal B: Environ 165(6):87–699. https://doi.org/10.1016/j.apcatb.2014.10.073
Comninellis C, Kapalka A, Malato S, Parsons SA, Poulios I, Mantzavinos D (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 83:769–776. https://doi.org/10.1002/jctb.1873
Costa RC, Moura FC, Ardisson J, Fabris J, Lago R (2008) Highly active heterogeneous Fenton-like systems based on Fe0/Fe3O4 composites prepared by controlled reduction of iron oxides. Appl Catal B: Environ 83:131–139. https://doi.org/10.1016/j.apcatb.2008.01.039
de la Plata GBO, Alfano OM, Cassano AE (2010) Decomposition of 2-chlorophenol employing goethite as Fenton catalyst. I. Proposal of a feasible, combined reaction scheme of heterogeneous and homogeneous reactions. Appl Catal B: Environ 95:1–13. https://doi.org/10.1016/j.apcatb.2009.12.005
Deng Y, Englehardt JD (2006) Treatment of landfill leachate by the Fenton process. Water Res 40:3683–3694. https://doi.org/10.1016/j.watres.2006.08.009
Deng J, Jiang J, Zhang Y, Lin X, Du C, Xiong Y (2008) FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of Orange II. Appl Catal B: Environ 84:468–473. https://doi.org/10.1016/j.apcatb.2008.04.029
Deng J, Wen X, Wang Q (2012) Solvothermal in situ synthesis of Fe3O4-multi-walled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Mater Res Bullet 47:3369–3376. https://doi.org/10.1016/j.materresbull.2012.07.021
Dhakshinamoorthy A, Navalon S, Alvaro M, Garcia H (2012) Metal nanoparticles as heterogeneous Fenton catalysts. Chemsuschem 5:46–64. https://doi.org/10.1002/cssc.201100517
Divya T, Renuka N (2015) Modulated heterogeneous Fenton-like activity of ‘M’doped nanoceria systems (M= Cu, Fe, Zr, Dy, La): Influence of reduction potential of doped cations. J Mol Catal A: Chem 408:41–47. https://doi.org/10.1016/j.molcata.2015.07.018
Do QC, Kim D-G, Ko S-O (2018) Catalytic activity enhancement of a Fe3O4@ SiO2 yolk-shell structure for oxidative degradation of acetaminophen by decoration with copper. J Clean Prod 172:1243–1253. https://doi.org/10.1016/j.jclepro.2017.10.246
Duarte F, Maldonado-Hodar F, Madeira LM (2012) Influence of the particle size of activated carbons on their performance as Fe supports for developing Fenton-like catalysts. Ind Eng Chem Res 51:9218–9226. https://doi.org/10.1021/ie300167r
Dükkancı M, Gündüz G, Yılmaz S, Yaman Y, Prikhod’Ko R, Stolyarova I (2010) Characterization and catalytic activity of CuFeZSM-5 catalysts for oxidative degradation of Rhodamine 6G in aqueous solutions. Appl Catal B: Environ 95:270–278. https://doi.org/10.1016/j.apcatb.2010.01.004
Fakhru Razi A, Pendashteh A, Abdullah LC, Biak DRA, Madaeni SS, Abidin ZZ (2009) Review of technologies for oil and gas produced water treatment. J Hazard Mater 170:530–551. https://doi.org/10.1016/j.jhazmat.2009.05.044
Fathy NA, El-Shafey SE, El-Shafey OI, Mohamed WS (2013) Oxidative degradation of RB19 dye by a novel γ-MnO2/MWCNT nanocomposite catalyst with H2O2. J Environ Chem Eng 1:858–864. https://doi.org/10.1016/j.jece.2013.07.028
Feng Y, Wu DL, Duan D, Lu MM Fenton-like oxidation of refractory chemical wastewater using pyrite. In: Advanced Materials Research Trans Tech Publ, 518: 2518–2525 https://doi.org/10.4028/www.scientific.net/AMR.518-523.2518
Feng Y, Wu D, Ma L (2013) Iron oxide catalyzed Fenton-like reaction. Prog Chem 25:1219. https://doi.org/10.7536/PC121143
Fenton H (1894) LXXIII.—Oxidation of tartaric acid in presence of iron Journal of the Chemical Society. Transactions 65:899–910. https://doi.org/10.1039/CT8946500899
Ferrizz R, Wong G, Egami T, Vohs J (2001) Structure sensitivity of the reaction of methanol on ceria. Langmuir 17:2464–2470. https://doi.org/10.1021/la001729o
Fink L, Dror I, Berkowitz B (2012) Enrofloxacin oxidative degradation facilitated by metal oxide nanoparticles. Chemosphere 86:144–149. https://doi.org/10.1016/j.chemosphere.2011.10.002
Flores Y, Flores R, Gallegos AA (2008) Heterogeneous catalysis in the Fenton-type system reactive black 5/ H2O2. J Mol Catal A: Chem 281:184–191. https://doi.org/10.1016/j.molcata.2007.10.019
Gao P, Chen X, Hao M, Xiao F, Yang S (2019) Oxygen vacancy enhancing the Fe2O3-CeO2 catalysts in Fenton-like reaction for the sulfamerazine degradation under O2 atmosphere. Chemosphere 228:521–527. https://doi.org/10.1016/j.chemosphere.2019.04.125
Garrido-Ramírez E, Theng B, Mora M (2010) Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—a review. Appl Clay Sci 47:182–192. https://doi.org/10.1016/j.clay.2009.11.044
Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol 32:147–156. https://doi.org/10.1016/j.nbt.2014.01.001
Geçgel Ü, Kocabıyık B, Üner O (2015) Adsorptive removal of methylene blue from aqueous solution by the activated carbon obtained from the fruit of catalpa bignonioides Water. Air, Soil Pollut 226:238. https://doi.org/10.1007/s11270-015-2513-4
Glassmeyer ST et al (2005) Transport of chemical and microbial compounds from known wastewater discharges: potential for use as indicators of human fecal contamination. Environ Sci Technol 39:5157–5169. https://doi.org/10.1021/es048120k
Glaze WH, Kang JW (1989) Advanced oxidation processes. Test of a kinetic model for the oxidation of organic compounds with ozone and hydrogen peroxide in a semibatch reactor. Ind Eng Chem Res 28:1580–1587. https://doi.org/10.1021/ie00095a002
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv Environ Res 8:501–551. https://doi.org/10.1016/S1093-0191(03)00032-7
Goldstein S, Meyerstein D, Czapski G (1993) The fenton reagents. Free Radic Biol Med 15:435–445. https://doi.org/10.1016/0891-5849(93)90043-T
Gonzalez-Olmos R, Holzer F, Kopinke F-D, Georgi A (2011) Indications of the reactive species in a heterogeneous Fenton-like reaction using Fe-containing zeolites. Appl Catal A 398:44–53. https://doi.org/10.1016/j.apcata.2011.03.005
Gumy D, Fernández-Ibáñez P, Malato S, Pulgarin C, Enea O, Kiwi J (2005) Supported Fe/C and Fe/Nafion/C catalysts for the photo-Fenton degradation of Orange II under solar irradiation. Catal Today 101:375–382. https://doi.org/10.1016/j.cattod.2005.03.036
Hammouda SB, Zhao F, Safaei Z, Babu I, Ramasamy DL, Sillanpää M (2017) Reactivity of novel Ceria-Perovskite composites CeO2-LaMO3 (MCu, Fe) in the catalytic wet peroxidative oxidation of the new emergent pollutant ‘Bisphenol F’: Characterization, kinetic and mechanism studies. Appl Catal B: Environ 218:119–136. https://doi.org/10.1016/j.apcatb.2017.06.047
Hamoud HI, Finqueneisel G, Azambre B (2017) Removal of binary dyes mixtures with opposite and similar charges by adsorption, coagulation/flocculation and catalytic oxidation in the presence of CeO2/H2O2 Fenton-like system. J Environ Manage 195:195–207. https://doi.org/10.1016/j.jenvman.2016.07.067
Hanna K, Kone T, Medjahdi G (2008) Synthesis of the mixed oxides of iron and quartz and their catalytic activities for the Fenton-like oxidation. Catal Commun 9:955–959. https://doi.org/10.1016/j.catcom.2007.09.035
Hartmann M, Kullmann S, Keller H (2010) Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J Mater Chem 20:9002–9017. https://doi.org/10.1039/C0JM00577K
Hassan H, Hameed B (2011a) Fe–clay as effective heterogeneous Fenton catalyst for the decolorization of Reactive Blue 4. Chem Eng J 171:912–918. https://doi.org/10.1016/j.cej.2011.04.040
Hassan H, Hameed B (2011b) Oxidative decolorization of Acid Red 1 solutions by Fe–zeolite Y type catalyst. Desalination 276:45–52. https://doi.org/10.1016/j.desal.2011.03.018
He W, Zhou Y-T, Wamer WG, Boudreau MD, Yin J-J (2012) Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 33:7547–7555. https://doi.org/10.1016/j.biomaterials.2012.06.076
He J, Yang X, Men B, Wang D (2016) Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J Environ Sci 39:97–109. https://doi.org/10.1016/j.jes.2015.12.003
Heckert EG, Karakoti AS, Seal S, Self WT (2008a) The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29:2705–2709. https://doi.org/10.1016/j.biomaterials.2008.03.014
Heckert EG, Seal S, Self WT (2008b) Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ Sci Technol 42:5014–5019. https://doi.org/10.1021/es8001508
Hermanek M, Zboril R, Medrik I, Pechousek J, Gregor C (2007) Catalytic efficiency of iron (III) oxides in decomposition of hydrogen peroxide: competition between the surface area and crystallinity of nanoparticles. J Am Chem Soc 129:10929–10936. https://doi.org/10.1021/ja072918x
Herney-Ramirez J, Lampinen M, Vicente MA, Costa CA, Madeira LM (2008) Experimental design to optimize the oxidation of Orange II dye solution using a clay-based Fenton-like catalyst. Ind Eng Chem Res 47:284–294. https://doi.org/10.1021/ie070990y
Huang C, Dong C, Tang Z (1993) Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manage 13:361–377. https://doi.org/10.1016/0956-053X(93)90070-D
Huanosta-Gutiérrez T, Dantas RF, Ramírez-Zamora R, Esplugas S (2012) Evaluation of copper slag to catalyze advanced oxidation processes for the removal of phenol in water. J Hazard Mater 213:325–330. https://doi.org/10.1016/j.jhazmat.2012.02.004
Hussain S, Aneggi E, Briguglio S, Mattiussi M, Gelao V, Cabras I, Zorzenon L, Trovarelli A, Goi D (2020) Enhanced ibuprofen removal by heterogeneous-Fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts. J Environ Chem Eng 8:103586. https://doi.org/10.1016/j.jece.2019.103586
Ioannou L, Puma GL, Fatta-Kassinos D (2015) Treatment of winery wastewater by physicochemical, biological and advanced processes: a review. J Hazard Mater 286:343–368. https://doi.org/10.1016/j.jhazmat.2014.12.043
Jain B, Singh AK, Kim H, Lichtfouse E, Sharma VK (2018) Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ Chem Lett 16:947–967. https://doi.org/10.1007/s10311-018-0738-3
Ji P, Wang L, Chen F, Zhang J (2010) Ce3+-centric organic pollutant elimination by CeO2 in the presence of H2O2. ChemCatChem 2:1552–1554. https://doi.org/10.1002/cctc.201000191
Jiang DB, Yuan Y, Zhao D, Tao K, Xu X, Zhang YX (2018) Facile synthesis of three-dimensional diatomite/manganese silicate nanosheet composites for enhanced Fenton-like catalytic degradation of malachite green dye. J Nanopart Res 20:123. https://doi.org/10.1007/s11051-018-4226-2
Jin Q, Kang J, Chen Q, Shen J, Guo F, Chen Z (2020) Efficiently enhanced Fenton-like reaction via Fe complex immobilized on silica particles for catalytic hydrogen peroxide degradation of 2, 4-dichlorophenol. Appl Catal B: Environ 268:118453. https://doi.org/10.1016/j.apcatb.2019.118453
John EM, Shaike JM (2015) Chlorpyrifos: pollution and remediation. Environ Chem Lett 13:269–291. https://doi.org/10.1007/s10311-015-0513-7
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales. UK Water research 42:3498–3518. https://doi.org/10.1016/j.watres.2008.04.026
Kim SD, Cho J, Kim IS, Vanderford BJ, Snyder SA (2007) Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res 41:1013–1021. https://doi.org/10.1016/j.watres.2006.06.034
Kim E-J, Oh D, Lee C-S, Gong J, Kim J, Chang Y-S (2017) Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: Crystal phase-dependent behavior. Catal Today 282:71–76. https://doi.org/10.1016/j.cattod.2016.03.034
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999−2000: A national reconnaissance Environmental science & technology 36:1202–1211. https://doi.org/https://doi.org/10.1021/es011055j
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B: Environ 49:1–14. https://doi.org/10.1016/j.apcatb.2003.11.010
Kundakovic L, Flytzani-Stephanopoulos M (1999) Deep oxidation of methane over zirconia supported Ag catalysts. Appl Catal A 183:35–51. https://doi.org/10.1016/S0926-860X(99)00043-5
Lahkimi A, Oturan MA, Oturan N, Chaouch M (2007) Removal of textile dyes from water by the electro-Fenton process. Environ Chem Lett 5:35–39. https://doi.org/10.1007/s10311-006-0058-x
Li Y, Zhang F-S (2010) Catalytic oxidation of Methyl Orange by an amorphous FeOOH catalyst developed from a high iron-containing fly ash. Chem Eng J 158:148–153. https://doi.org/10.1016/j.cej.2009.12.021
Li D, Yang T, Li Y, Liu Z, Jiao W (2020) Facile and green synthesis of highly dispersed tar-based heterogeneous Fenton catalytic nanoparticles for the degradation of methylene blue. J Clean Prod 246:119033. https://doi.org/10.1016/j.jclepro.2019.119033
Lin Z-R, Zhao L, Dong Y-H (2015) Quantitative characterization of hydroxyl radical generation in a goethite-catalyzed Fenton-like reaction. Chemosphere 141:7–12. https://doi.org/10.1016/j.chemosphere.2015.05.066
Liu X, Zhou K, Wang L, Wang B, Li Y (2009) Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J Am Chem Soc 131:3140–3141. https://doi.org/10.1021/ja808433d
Lucas MS, Peres JA (2009) Treatment of olive mill wastewater by a combined process: Fenton’s reagent and chemical coagulation. J Environ Sci Health Part A 44:198–205. https://doi.org/10.1080/10934520802539889
Luo M, Bowden D, Brimblecombe P (2009) Catalytic property of Fe-Al pillared clay for Fenton oxidation of phenol by H2O2. Appl Catal B: Environ 85:201–206. https://doi.org/10.1016/j.apcatb.2008.07.013
Luo W, Zhu L, Wang N, Tang H, Cao M, She Y (2010) Efficient removal of organic pollutants with magnetic nanoscaled BiFeO3 as a reusable heterogeneous Fenton-like catalyst. Environ Sci Technol 44:1786–1791. https://doi.org/10.1021/es903390g
Lv H, Zhao H, Cao T, Qian L, Wang Y, Zhao G (2015) Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J Mol Catal A: Chem 400:81–89. https://doi.org/10.1016/j.molcata.2015.02.007
Lyu L, Zhang L, Hu C (2015) Enhanced Fenton-like degradation of pharmaceuticals over framework copper species in copper-doped mesoporous silica microspheres. Chem Eng J 274:298–306. https://doi.org/10.1016/j.cej.2015.03.137
Mao C-F, Vannice MA (1995) High surface area α-aluminas III. Oxidation of ethylene, ethylene oxide, and acetaldehyde over silver dispersed on high surface area α-alumina. Appl Catal A: Gen 122:61–76. https://doi.org/10.1016/0926-860X(94)00214-2
Metz F, Ingold K (2014) Sustainable wastewater management: is it possible to regulate micropollution in the future by learning from the past? A Policy Anal Sustain 6:1992–2012. https://doi.org/10.3390/su6041992
Miles CJ, Brezonik PL (1981) Oxygen consumption in humic-colored waters by a photochemical ferrous-ferric catalytic cycle. Environ Sci Technol 15:1089–1095. https://doi.org/10.1021/es00091a010
Minghao S, Lei S, Sheng L, Jinjie W, Zhang L, Huang S (2013) Ordered mesoporous manganese oxide as catalyst for hydrogen peroxide oxidation of norfloxacin in water. Chinese J Catal 34:536–541. https://doi.org/10.1016/S1872-2067(11)60492-0
Molina R, Martínez F, Melero JA, Bremner DH, Chakinala AG (2006) Mineralization of phenol by a heterogeneous ultrasound/Fe-SBA-15/H2O2 process: multivariate study by factorial design of experiments. Appl Catal B: Environ 66:198–207. https://doi.org/10.1016/j.apcatb.2006.03.015
Mosteo R, Ormad M, Ovelleiro J (2007) Photo-Fenton processes assisted by solar light used as preliminary step to biological treatment applied to winery wastewaters. Water Sci Technol 56:89–94. https://doi.org/10.2166/wst.2007.476
Mousset E, Dionysiou DD (2020) Photoelectrochemical reactors for treatment of water and wastewater: a review. Environ Chem Lett 18:1301–1318. https://doi.org/10.1007/s10311-020-01014-9
Munoz M, de Pedro ZM, Menendez N, Casas JA, Rodriguez JJ (2013) A ferromagnetic γ-alumina-supported iron catalyst for CWPO Application to chlorophenols. Appl Catal B: Environ 136:218–224. https://doi.org/10.1016/j.apcatb.2013.02.002
Muthuvel I, Swaminathan M (2008) Highly solar active Fe (III) immobilised alumina for the degradation of Acid Violet 7. Solar Energy Mater Solar Cells 92:857–863. https://doi.org/10.1016/j.solmat.2008.02.007
Nasuha N, Ismail S, Hameed B (2016) Activated electric arc furnace slag as an efficient and reusable heterogeneous Fenton-like catalyst for the degradation of Reactive Black 5. J Taiwan Inst Chem Eng 67:235–243. https://doi.org/10.1016/j.jtice.2016.07.023
Navalon S, Alvaro M, Garcia H (2010) Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl Catal B: Environ 99:1–26. https://doi.org/10.1002/cssc.201100216
Navalon S, Dhakshinamoorthy A, Alvaro M, Garcia H (2011) Heterogeneous Fenton catalysts based on activated carbon and related materials. Chemsuschem 4:1712–1730. https://doi.org/10.1016/j.apcatb.2010.07.006
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50. https://doi.org/10.1016/S0304-3894(02)00282-0
Nidheesh P (2015) Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review. Rsc Adv 5:40552–40577. https://doi.org/10.1039/C5RA02023A
Nidheesh PV, Gandhimathi R, Ramesh ST (2013) Degradation of dyes from aqueous solution by Fenton processes: a review. Environ Sci Pollut Res 20:2099–2132. https://doi.org/10.1007/s11356-012-1385-z
Noyes AA, Whitney WR (1897) The rate of solution of solid substances in their own solutions. J Am Chem Soc 19:930–934. https://doi.org/10.1021/ja02086a003
Oller I, Malato S, Sánchez-Pérez J (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166. https://doi.org/10.1016/j.scitotenv.2010.08.061
Othman I, Haija MA, Ismail I, Zain JH, Banat F (2019) Preparation and catalytic performance of CuFe2O4 nanoparticles supported on reduced graphene oxide (CuFe2O4/rGO) for phenol degradation. Mater Chem Phys 238:121931. https://doi.org/10.1016/j.matchemphys.2019.121931
Otsuka M, Tanabe H, Osaki K, Otsuka K, Ozaki Y (2007) Chemoinformetrical evaluation of dissolution property of indomethacin tablets by near-infrared spectroscopy. J Pharm Sci 96:788–801. https://doi.org/10.1002/jps.20704
Pachamuthu MP, Karthikeyan S, Maheswari R, Lee AF, Ramanathan A (2017) Fenton-like degradation of Bisphenol A catalyzed by mesoporous Cu/TUD-1. Appl Surf Sci 393:67–73. https://doi.org/10.1016/j.apsusc.2016.09.162
Paramo-Vargas J, Granados SG, Maldonado-Rubio MI, Peralta-Hernandez JM (2016) Up to 95 % reduction of chemical oxygen demand of slaughterhouse effluents using Fenton and photo-Fenton oxidation. Environ Chem Lett 14:149–154. https://doi.org/10.1007/s10311-015-0534-2
Parida K, Dash S, Mallik S, Das J (2005) Effect of heat treatment on the physico-chemical properties and catalytic activity of manganese nodules leached residue towards decomposition of hydrogen peroxide. J Colloid Interface Sci 290:431–436. https://doi.org/10.1016/j.jcis.2005.04.056
Park CM, Heo J, Yoon Y (2017) Oxidative degradation of bisphenol A and 17α-ethinyl estradiol by Fenton-like activity of silver nanoparticles in aqueous solution. Chemosphere 168:617–622. https://doi.org/10.1016/j.chemosphere.2016.11.016
Perathoner S, Centi G (2005) Wet hydrogen peroxide catalytic oxidation (WHPCO) of organic waste in agro-food and industrial streams. Topics Catal 33:207–224. https://doi.org/10.1007/s11244-005-2529-x
Pereira M, Oliveira L, Murad E (2012) Iron oxide catalysts: Fenton and Fentonlike reactions–a review. Clay Miner 47:285–302. https://doi.org/10.1180/claymin.2012.047.3.01
Pirsaheb M, Moradi S, Shahlaei M, Wang X, Farhadian N (2019) A new composite of nano zero-valent iron encapsulated in carbon dots for oxidative removal of bio-refractory antibiotics from water. J Clean Prod 209:1523–1532. https://doi.org/10.1016/j.jclepro.2018.11.175
Pouran SR, Raman AAA, Daud WMAW (2014) Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J Clean Prod 64:24–35. https://doi.org/10.1016/j.jclepro.2013.09.013
Qu Z, Cheng M, Huang W, Bao X (2005) Formation of subsurface oxygen species and its high activity toward CO oxidation over silver catalysts. J Catal 229:446–458. https://doi.org/10.1016/j.jcat.2004.11.043
Quinn B, Gagné F, Blaise C (2008) An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian Hydra attenuata. Sci Total Environ 389:306–314. https://doi.org/10.1016/j.scitotenv.2007.08.038
Rayaroth MP, Aravind UK, Aravindakumar CT (2016) Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ Chem Lett 14:259–290. https://doi.org/10.1007/s10311-016-0568-0
Ren Y, Ma Z, Bruce PG (2012) Ordered mesoporous metal oxides: synthesis and applications. Chem Soc Rev 41:4909–4927. https://doi.org/10.1039/C2CS35086F
Rhadfi T, Piquemal J-Y, Sicard L, Herbst F, Briot E, Benedetti M, Atlamsani A (2010) Polyol-made Mn3O4 nanocrystals as efficient Fenton-like catalysts. Appl Catal A: Gen 386:132–139. https://doi.org/10.1016/j.apcata.2010.07.044
Rigg T, Taylor W, Weiss J (1954) The rate constant of the reaction between hydrogen peroxide and ferrous ions. J Chem Phys 22:575–577. https://doi.org/10.1063/1.1740127
Robinson DM et al (2013) Photochemical water oxidation by crystalline polymorphs of manganese oxides: structural requirements for catalysis. J Am Chem Soc 135:3494–3501. https://doi.org/10.1021/ja310286h
Rossi AF, Amaral-Silva N, Martins RC, Quinta-Ferreira RM (2012) Heterogeneous Fenton using ceria based catalysts: effects of the calcination temperature in the process efficiency. Appl Catal B 111:254–263. https://doi.org/10.1016/j.apcatb.2011.10.006
Rueda Márquez JJ, Levchuk I, Sillanpää M (2018) Application of catalytic wet peroxide oxidation for industrial and urban wastewater treatment: a review. Catalysts 8:673. https://doi.org/10.3390/catal8120673
Rusevova K, Kopinke F-D, Georgi A (2012) Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions—Influence of Fe (II)/Fe (III) ratio on catalytic performance. J Hazard Mater 241:433–440. https://doi.org/10.1016/j.jhazmat.2012.09.068
Rusevova K, Köferstein R, Rosell M, Richnow HH, Kopinke F-D, Georgi A (2014) LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions. Chem Eng J 239:322–331. https://doi.org/10.1016/j.cej.2013.11.025
Saeed M, Ahmad A, Boddula R, Ul Haq A, Azhar A (2018) Ag@MnxOy: an effective catalyst for photo-degradation of rhodamine B dye. Environ Chem Lett 16:287–294. https://doi.org/10.1007/s10311-017-0661-z
Salazar R, Brillas E, Sirés I (2012) Finding the best Fe2+/Cu2+ combination for the solar photoelectro-Fenton treatment of simulated wastewater containing the industrial textile dye Disperse Blue 3. Appl Catal B: Environ 115:107–116. https://doi.org/10.1016/j.apcatb.2011.12.026
Salem IA (2000) Kinetics of the oxidative color removal and degradation of bromophenol blue with hydrogen peroxide catalyzed by copper (II)-supported alumina and zirconia. Appl Catal B: Environ 28:153–162. https://doi.org/10.1016/S0926-3373(00)00173-9
Salimi M et al (2017) Contaminants of emerging concern: a review of new approach in AOP technologies. Environ Monit Assess 189:414. https://doi.org/10.1007/s10661-017-6097-x
Shahidi D, Roy R, Azzouz A (2015) Advances in catalytic oxidation of organic pollutants–prospects for thorough mineralization by natural clay catalysts. Appl Catal B: Environ 174:277–292. https://doi.org/10.1016/j.apcatb.2015.02.042
Shen Y, Zhang Z, Xiao K (2015) Evaluation of cobalt oxide, copper oxide and their solid solutions as heterogeneous catalysts for Fenton-degradation of dye pollutants. RSC Adv 5:91846–91854. https://doi.org/10.1039/C5RA18923C
Shen Y, Zhou Y, Zhang Z, Xiao K (2017) Cobalt–copper oxalate nanofibers mediated Fenton degradation of Congo red in aqueous solutions. J Ind Eng Chem 52:153–161. https://doi.org/10.1016/j.jiec.2017.03.038
Sigel H (2000) Metal ions in biological systems: volume 37: manganese and its role in biological processes. CRC Press. https://doi.org/10.1021/jm000216b
Singh L, Rekha P, Chand S (2016) Cu-impregnated zeolite Y as highly active and stable heterogeneous Fenton-like catalyst for degradation of Congo red dye. Sep Purif Technol 170:321–336. https://doi.org/10.1016/j.seppur.2016.06.059
Sirés I, Garrido JA, Rodríguez RM, Centellas F, Arias C, Brillas E (2006) Electrochemical degradation of paracetamol from water by catalytic action of Fe2+, Cu2+, and UVA light on electrogenerated hydrogen peroxide. J Electrochem Soc 153:D1–D9. https://doi.org/10.1149/1.2130568
Soares PA, Silva TF, Manenti DR, Souza SM, Boaventura RA, Vilar VJ (2014) Insights into real cotton-textile dyeing wastewater treatment using solar advanced oxidation processes. Environ Sci Pollut Res 21:932–945. https://doi.org/10.1007/s11356-013-1934-0
Song H et al (2016) Manganese functionalized mesoporous molecular sieves Ti-HMS as a Fenton-like catalyst for dyes wastewater purification by advanced oxidation processes Journal of Environmental. Chem Eng 4:4653–4660. https://doi.org/10.1016/j.jece.2016.09.039
Sun Q et al (2017) Synthesis of Fe/M (M= Mn Co, Ni) bimetallic metal organic frameworks and their catalytic activity for phenol degradation under mild conditions. Inorg Chem Front 4:144–153. https://doi.org/10.1039/C6QI00441E
Sun Y et al (2019) Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Appl Catal B 258:117985. https://doi.org/10.1016/j.apcatb.2019.117985
Tang Y, Ren H, Yang P, Li H, Zhang J, Qu C, Chen G (2019) Treatment of fracturing fluid waste by Fenton reaction using transition metal complexes catalyzes oxidation of hydroxypropyl guar gum at high pH. Environ Chem Lett 17:559–564. https://doi.org/10.1007/s10311-018-0805-9
Tian S, Tu Y, Chen D, Chen X, Xiong Y (2011) Degradation of Acid Orange II at neutral pH using Fe2(MoO4)3 as a heterogeneous Fenton-like catalyst. Chem Eng J 169:31–37. https://doi.org/10.1016/j.cej.2011.02.045
Tian X, Jin H, Nie Y, Zhou Z, Yang C, Li Y, Wang Y (2017) Heterogeneous Fenton-like degradation of ofloxacin over a wide pH range of 3.6–10.0 over modified mesoporous iron oxide. Chem Eng J 328:397–405. https://doi.org/10.1016/j.cej.2017.07.049
Tiya-Djowe A, Nzali S, Njoyim ET, Laminsi S, Gaigneaux EM (2016) Thermal treatment of plasma-synthesized goethite improves Fenton-like degradation of orange II dye. Environ Chem Lett 14:515–519. https://doi.org/10.1007/s10311-016-0578-y
Trovarelli A, de Leitenburg C, Boaro M, Dolcetti G (1999) The utilization of ceria in industrial catalysis. Catal Today 50:353–367. https://doi.org/10.1016/S0920-5861(98)00515-X
Tušar NN et al (2012) Manganese functionalized silicate nanoparticles as a fenton-type catalyst for water purification by advanced oxidation processes (AOP). Adv Funct Mater 22:820–826. https://doi.org/10.1002/adfm.201102361
Umar M, Aziz HA, Yusoff MS (2010) Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manage 30:2113–2121. https://doi.org/10.1016/j.wasman.2010.07.003
Vindedahl AM, Strehlau JH, Arnold WA, Penn RL (2016) Organic matter and iron oxide nanoparticles: aggregation, interactions, and reactivity. Environ Sci: Nano 3:494–505. https://doi.org/10.1039/C5EN00215J
Walling C (1975) Fenton’s reagent revisited. Acc Chem Res 8:125–131. https://doi.org/10.1021/ar50088a003
Walling C, Goosen A (1973) Mechanism of the ferric ion catalyzed decomposition of hydrogen peroxide Effect of organic substrates. J Am Chem Soc 95:2987–2991. https://doi.org/10.1021/ja00790a042
Wang J, Zhuan R (2020) Degradation of antibiotics by advanced oxidation processes: an overview. Sci Total Environ 701:135023. https://doi.org/10.1016/j.scitotenv.2019.135023
Wang Y, Zhao H, Zhao G, Wang Y, Yang X (2013) Iron compound-based heterogeneous Fenton catalytic oxidation technology. Prog Chem 25:1246. https://doi.org/10.7536/PC121201
Wang Y, Zhao H, Zhao G (2015) Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl Catal B 164:396–406. https://doi.org/10.1016/j.apcatb.2014.09.047
Wang N, Zheng T, Zhang G, Wang P (2016) A review on Fenton-like processes for organic wastewater treatment Journal of Environmental. Chem Eng 4:762–787. https://doi.org/10.1016/j.jece.2015.12.016
Wang N, Hao L, Chen J, Zhao Q, Xu H (2018) Adsorptive removal of organics from aqueous phase by acid-activated coal fly ash: preparation, adsorption, and Fenton regenerative valorization of “spent” adsorbent. Environ Sci Pollut Res 25:12481–12490. https://doi.org/10.1007/s11356-018-1560-y
Watts RJ, Sarasa J, Loge FJ, Teel AL (2005) Oxidative and reductive pathways in manganese-catalyzed Fenton’s reactions. J Environ Eng 131:158–164. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:1(158)
Weaver J, Frederikse H (1977) Crc handbook of chemistry and physics. CRC Press, Boca Raton 76:12-156 https://doi.org/10.1021/ja069813z
Wei X, Xie X, Wang Y, Yang S (2020) Shape-Dependent Fenton-Like Catalytic Activity of Fe3O4 Nanoparticles. J Environ Eng 146:04020005. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001648
Wu J, Lin G, Li P, Yin W, Wang X, Yang B (2013) Heterogeneous Fenton-like degradation of an azo dye reactive brilliant orange by the combination of activated carbon–FeOOH catalyst and H2O2. Water Sci Technol 67:572–578. https://doi.org/10.2166/wst.2012.596
Wu X, Xia F, Nan Z (2020) Facile synthesis of double-mesoporous-shelled hollow spheres of Cu–CuFe2O4/SiO2 composite as excellent Fenton catalyst. Mater Chem Phys 242:122490. https://doi.org/10.1016/j.matchemphys.2019.122490
Xia M, Long M, Yang Y, Chen C, Cai W, Zhou B (2011) A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl Catal B: Environ 110:118–125. https://doi.org/10.1016/j.apcatb.2011.08.033
Xiao W, Wang D, Lou XW (2010) Shape-controlled synthesis of MnO2 nanostructures with enhanced electrocatalytic activity for oxygen reduction. J Phys Chem C 114:1694–1700. https://doi.org/10.1021/jp909386d
Xie F, Zhong J, Wang L, Wang K, Hua D (2015) Degradation of acid scarlet 3R with CuO/SiO2 hollow sphere catalyst. In: IOP Conference Series: Materials Science and Engineering, 1: 012039 https://doi.org/10.1088/1757-899X/87/1/012039
Xie H, Zeng J, Zhou G (2020) CeCu composite oxide for chlorophenol effective removal by heterogeneous catalytic wet peroxide oxidation. Environ Sci Pollut Res 27:846–860. https://doi.org/10.1007/s11356-019-07042-5
Xu L, Wang J (2011) A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J Hazard Mater 186:256–264. https://doi.org/10.1016/j.jhazmat.2010.10.116
Xu L, Wang J (2012) Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ Sci Technol 46:10145–10153. https://doi.org/10.1021/es300303f
Xu L, Wang J (2015) Degradation of 2, 4, 6-trichlorophenol using magnetic nanoscaled Fe3O4/CeO2 composite as a heterogeneous Fenton-like catalyst. Sep Purif Technol 149:255–264. https://doi.org/10.1016/j.seppur.2015.05.011
Xu N, Guo D, Xiao C (2019) Fe/Mn oxide decorated polyacrylonitrile hollow fiber membrane as heterogeneous Fenton reactor for methylene blue decolorization. J Appl Polym Sci 136:48217. https://doi.org/10.1002/app.48217
Xu Y, Guo X, Zha F, Tang X, Tian H (2020) Efficient photocatalytic removal of orange II by a Mn3O4–FeS2/Fe2O3 heterogeneous catalyst. J Environ Manage 253:109695. https://doi.org/10.1016/j.jenvman.2019.109695
Xue X, Hanna K, Deng N (2009) Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J Hazard Mater 166:407–414. https://doi.org/10.1016/j.jhazmat.2008.11.089
Yaman YC, Gündüz G, Dükkancı M (2013) Degradation of CI R eactive R ed 141 by heterogeneous F enton‐like process over iron‐containing ZSM‐5 zeolites. Coloration Technology 129: 69–75 https://doi.org/10.1111/cote.12001
Yang B, Tian Z, Zhang L, Guo Y, Yan S (2015) Enhanced heterogeneous Fenton degradation of Methylene Blue by nanoscale zero valent iron (nZVI) assembled on magnetic Fe3O4/reduced graphene oxide. J Water Process Eng 5:101–111. https://doi.org/10.1016/j.jwpe.2015.01.006
Yang X, He J, Yang Q, Jiao R, Liao G, Wang D (2019) Cu (I)-doped Fe3O4 nanoparticles/porous C composite for enhanced H2O2 oxidation of carbamazepine. J Colloid Interface Sci 551:16–25. https://doi.org/10.1016/j.jcis.2019.04.083
Zha S, Cheng Y, Gao Y, Chen Z, Megharaj M, Naidu R (2014) Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem Eng J 255:141–148. https://doi.org/10.1016/j.cej.2014.06.057
Zhang X, Ding Y, Tang H, Han X, Zhu L, Wang N (2014) Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: efficiency, stability and mechanism. Chem Eng J 236:251–262. https://doi.org/10.1016/j.cej.2013.09.051
Zhang N, Chen J, Fang Z, Tsang EP (2019) Ceria accelerated nanoscale zerovalent iron assisted heterogenous Fenton oxidation of tetracycline. Chem Eng J 369:588–599. https://doi.org/10.1016/j.cej.2019.03.112
Zhang N, Tsang EP, Chen J, Fang Z, Zhao D (2020a) Critical role of oxygen vacancies in heterogeneous Fenton oxidation over ceria-based catalysts. J Colloid Interface Sci 558:163–172. https://doi.org/10.1016/j.jcis.2019.09.079
Zhang N, Yi Y, Lian J, Fang Z (2020b) Effects of Ce doping on the Fenton-like reactivity of Cu-based catalyst to the fluconazole. Chem Eng J 395:124897. https://doi.org/10.1016/j.cej.2020.124897
Zhao G, Li J, Ren X, Hu J, Hu W, Wang X (2013) Highly active MnO2 nanosheet synthesis from graphene oxide templates and their application in efficient oxidative degradation of methylene blue. RSC Adv 3:12909–12914. https://doi.org/10.1039/C3RA40942B
Zhou T, Li Y, Ji J, Wong F-S, Lu X (2008) Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: Kinetic, pathway and effect factors. Sep Purif Technol 62:551–558. https://doi.org/10.1016/j.seppur.2008.03.008
Zubir NA, Yacou C, Motuzas J, Zhang X, Da Costa JCD (2014) Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci Rep 4:4594. https://doi.org/10.1038/srep04594
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Open Access funding provided by Università degli Studi di Udine. S.H. performed the literature search and data analysis and drafted the work. E. A. had the idea for the article and critically revised the work. D. G. had the idea for the article and revised the work.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussain, S., Aneggi, E. & Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: a review. Environ Chem Lett 19, 2405–2424 (2021). https://doi.org/10.1007/s10311-021-01185-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01185-z