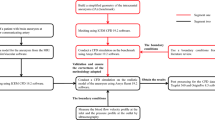
Abstract
The abdominal aorta is the largest artery in the abdominal cavity that supplies blood flows to vital organs through the complex visceral arterial branches, including the celiac trunk (the liver, stomach, spleen, etc.), the renal arteries (the kidneys) and the superior and inferior mesenteric arteries (the small and large intestine, pancreas, etc.). An accurate simulation of blood flows in this network of arteries is important for the understanding of the hemodynamics in various organs of healthy and diseased patients, but the computational cost is very high. As a result, most researchers choose to focus on a portion of the artery or use a low-dimensional approximation of the artery. In the present work, we introduce a parallel algorithm for the modeling of pulsatile flows in the abdominal aorta with branches to the primary organs, and an organ-based two-level method for calculating the resistances for the outflow boundary conditions. With this highly parallel approach, the simulation of the blood flow for a cardiac cycle of the anatomically detailed aorta can be obtained within a few hours, and the blood distribution to organs including liver, spleen and kidneys are also computed with certain accuracy. Moreover, we discuss the significant hemodynamic differences resulted from the influence of the peripheral branches. In addition, we examine the accuracy of the results with respect to the mesh size and time-step size and show the high parallel scalability of the proposed algorithm with up to 3000 processor cores.













Similar content being viewed by others
References
Ambler G, Coughlin P, Hayes P, Varty K, Gohel M, Boyle J (2015) Incidence and outcomes of severe renal impairment following ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 50(4):443–449
Balay S, Abhyankar S, Adams MF, Brown J, Brune P, Buschelman K, Dalcin L, Dener A, Eijkhout V, Gropp W et al. (2020) PETSc Users Manual revision 3.13. Technical report, Argonne National Lab.(ANL), Argonne, IL
Barker AT, Cai X-C (2010) Scalable parallel methods for monolithic coupling in fluid-structure interaction with application to blood flow modeling. J Comput Phys 229(3):642–659
Blanco P, Watanabe S, Feijóo R (2012) Identification of vascular territory resistances in one-dimensional hemodynamics simulations. J Biomech 45(12):2066–2073
Blanco PJ, Müller LO, Watanabe SM, Feijóo RA (2020) On the anatomical definition of arterial networks in blood flow simulations: comparison of detailed and simplified models. Biomech Model Mechan 19(5):1663–1678
Blanco PJ, Watanabe SM, Dari EA, Passos MAR, Feijóo RA (2014) Blood flow distribution in an anatomically detailed arterial network model: criteria and algorithms. Biomech Model Mechan 13(6):1303–1330
Boyd AJ, Kuhn DC, Lozowy RJ, Kulbisky GP (2016) Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg 63(6):1613–1619
Capoccia M (2015) Development and characterization of the arterial windkessel and its role during left ventricular assist device assistance. Artif Organs 39(8):E138–E153
Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL et al (2018) The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 67(1):2–77
Chisci E, Alamanni N, Iacoponi F, Michelagnoli S, Procacci T, Colombo G, Setacci C (2018) Grading abdominal aortic aneurysm rupture risk. J Card Surg 59(1):87–94
Chung B, Cebral JR (2015) CFD for evaluation and treatment planning of aneurysms: review of proposed clinical uses and their challenges. Ann Biomed Eng 43(1):122–138
Figueroa CA, Humphrey JD (2014) Pressure wave propagation in full-body arterial models: a gateway to exploring aging and hypertension. Procedia IUTAM 10:382–395
Fossan FE, Sturdy J, Müller LO, Strand A, Bråten AT, Jørgensen A, Wiseth R, Hellevik LR (2018) Uncertainty quantification and sensitivity analysis for computational FFR estimation in stable coronary artery disease. Cardiovasc Eng Techn 9(4):597–622
Frauenfelder T, Lotfey M, Boehm T, Wildermuth S (2006) Computational fluid dynamics: hemodynamic changes in abdominal aortic aneurysm after stent-graft implantation. Cardiovasc Inter Rad 29(4):613–623
Ghulam Q, Bredahl K, Lönn L, Rouet L, Sillesen H, Eiberg J (2017) Follow-up on small abdominal aortic aneurysms using three dimensional ultrasound: volume versus diameter. Eur J Vasc Endovasc Surg 54:439–445
Grinberg L, Anor T, Madsen J, Yakhot A, Karniadakis G (2009) Large-scale simulation of the human arterial tree. Clin Exp Pharmacol Physiol 36(2):194–205
Grinberg L, Karniadakis GE (2008) Outflow boundary conditions for arterial networks with multiple outlets. Ann Biomech Eng 36(9):1496–1514
Kandail H, Hamady M, Xu XY (2015) Comparison of blood flow in branched and fenestrated stent-grafts for endovascular repair of abdominal aortic aneurysms. J Endovasc Ther 22(4):578–590
Keegan J, Patel HC, Simpson RM, Mohiaddin RH, Firmin DN (2015) Inter-study reproducibility of interleaved spiral phase velocity mapping of renal artery haemodynamics. J Cardiovasc Magn Reson 17(1):8
Kent KC (2014) Abdominal aortic aneurysms. N Engl J Med 371(22):2101–2108
Kong F, Kheyfets V, Finol E, Cai X-C (2018) An efficient parallel simulation of unsteady blood flows in patient-specific pulmonary artery. Int J Numer Meth Bio 34(4):e2952
Lan H, Updegrove A, Wilson NM, Maher GD, Shadden SC, Marsden AL (2018) A re-engineered software interface and workflow for the open-source Simvascular cardiovascular modeling package. J Biomech Eng 140(2):024501
Lee D, Chen J (2002) Numerical simulation of steady flow fields in a model of abdominal aorta with its peripheral branches. J Biomech 35(8):1115–1122
Lee D, Chen J (2003) Pulsatile flow fields in a model of abdominal aorta with its peripheral branches. Biomed Eng Appl Basis Commun 15(05):170–178
Lefferts WK, Augustine JA, Heffernan KS (2014) Effect of acute resistance exercise on carotid artery stiffness and cerebral blood flow pulsatility. Front Physiol 5:101
Les AS, Shadden SC, Figueroa CA, Park JM, Tedesco MM, Herfkens RJ, Dalman RL, Taylor CA (2010) Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann Biomed Eng 38(4):1288–1313
Li Z, Jiang W, Yuan D, Chen Y, Tian X, Zhou Z (2018) Investigation of the hemodynamics of a juxtarenal aortic aneurysm with intervention by dual-stents strategy. Clin Biomech 58:109–115
Liao Z-J, Chen R, Yan Z, Cai X-C (2019) A parallel implicit domain decomposition algorithm for the large eddy simulation of incompressible turbulent flows on 3D unstructured meshes. Int J Numer Meth Fl 89(9):343–361
Liu H, Liang F, Wong J, Fujiwara T, Ye W, Tsubota K-I, Sugawara M (2015) Multi-scale modeling of hemodynamics in the cardiovascular system. Acta Mech Sin 31(4):446–464
Liu J, Yan Z, Pu Y, Shiu W-S, Wu J, Chen R, Leng X, Qin H, Liu X, Jia B et al (2017) Functional assessment of cerebral artery stenosis: a pilot study based on computational fluid dynamics. J Cereb Blood Flow Metab 37(7):2567–2576
Lopes D, Puga H, Teixeira J, Teixeira S (2019) Influence of arterial mechanical properties on carotid blood flow: comparison of CFD and FSI studies. Int J Mech Sci 160:209–218
Luo L, Shiu W-S, Chen R, Cai X-C (2019) A nonlinear elimination preconditioned inexact newton method for blood flow problems in human artery with stenosis. J Comput Phys 399:108926
Morris PD, Narracott A, von Tengg-Kobligk H, Soto DAS, Hsiao S, Lungu A, Evans P, Bressloff NW, Lawford PV, Hose DR et al (2016) Computational fluid dynamics modelling in cardiovascular medicine. Heart 102(1):18–28
Nakamura T, Moriyasu F, Ban N, Nishida O, Tamada T, Kawasaki T, Sakai M, Uchino H (1989) Quantitative measurement of abdominal arterial blood flow using image-directed doppler ultrasonography: superior mesenteric, splenic, and common hepatic arterial blood flow in normal adults. J Clin Ultrasound 17(4):261–268
Owen B, Lowe C, Ashton N, Mandal P, Rogers S, Wein W, McCollum C, Revell A (2016) Computational hemodynamics of abdominal aortic aneurysms: three-dimensional ultrasound versus computed tomography. Proc Inst Mech Eng H 230(3):201–210
Regnier P, Lareyre F, Hassen-Khodja R, Durand M, Touma J, Raffort J (2018) Sexual dysfunction after abdominal aortic aneurysm surgical repair: current knowledge and future directions. Eur J Vasc Endovasc Surg 55(2):267–280
Sato S, Ohnishi K, Sugita S, Okuda K (1987) Splenic artery and superior mesenteric artery blood flow: nonsurgical Doppler us measurement in healthy subjects and patients with chronic liver disease. Radiology 164(2):347–352
Shipkowitz T, Rodgers V, Frazin LJ, Chandran K (1998) Numerical study on the effect of steady axial flow development in the human aorta on local shear stresses in abdominal aortic branches. J Biomech 31(11):995–1007
Shipkowitz T, Rodgers V, Frazin LJ, Chandran K (2000) Numerical study on the effect of secondary flow in the human aorta on local shear stresses in abdominal aortic branches. J Biomech 33(6):717–728
Taylor CA, Fonte TA, Min JK (2013) Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 61(22):2233–2241
Taylor CA, Hughes TJ, Zarins CK (1998) Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann Biomed Eng 26(6):975–987
Tse KM, Chiu P, Lee HP, Ho P (2011) Investigation of hemodynamics in the development of dissecting aneurysm within patient-specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J Biomech 44(5):827–836
Valentin J (2002) Basic anatomical and physiological data for use in radiological protection: reference values. Ann ICRP 32(3–4):1–277
Vignon-Clementel IE, Figueroa CA, Jansen KE, Taylor CA (2006) Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput Methods Appl Mech Eng 195(29–32):3776–3796
Xiao N (2014) Simulation of 3-D blood flow in the full systemic arterial tree and computational frameworks for efficient parameter estimation. PhD thesis, Stanford University
Xiao N, Alastruey J, Alberto Figueroa C (2014) A systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int J Numer Meth Bio 30(2):204–231
Xiong Y, Wang X, Jiang W, Tian X, Wang Q, Fan Y, Chen Y (2016) Hemodynamics study of a multilayer stent for the treatment of aneurysms. Biomed Eng online 15(2):134
Yang C, Cai X-C (2011) Parallel multilevel methods for implicit solution of shallow water equations with nonsmooth topography on the cubed-sphere. J Comput Phys 230(7):2523–2539
Yang C, Cai X-C (2014) A scalable fully implicit compressible Euler solver for mesoscale nonhydrostatic simulation of atmospheric flows. SIAM J Sci Comput 36(5):S23–S47
Youssefi P, Gomez A, Arthurs C, Sharma R, Jahangiri M, Alberto Figueroa C (2018) Impact of patient-specific inflow velocity profile on hemodynamics of the thoracic aorta. J Biomech Eng 140(1):011002
Yzet T, Bouzerar R, Allart J-D, Demuynck F, Legallais C, Robert B, Deramond H, Meyer M-E, Balédent O (2010) Hepatic vascular flow measurements by phase contrast MRI and Doppler echography: a comparative and reproducibility study. J Magn Reson Imaging 31(3):579–588
Zhang J, Critchley L, Huang L (2015) Five algorithms that calculate cardiac output from the arterial waveform: a comparison with Doppler ultrasound. Brit J Anaesth 115(3):392–402
Zhang R, Behbehani K, Levine BD (2009) Dynamic pressure-flow relationship of the cerebral circulation during acute increase in arterial pressure. J physiol 587(11):2567–2577
Zhou M, Sahni O, Kim HJ, Figueroa CA, Taylor CA, Shephard MS, Jansen KE (2010) Cardiovascular flow simulation at extreme scale. Comput Mech 46(1):71–82
Zhou S, Xu L, Hao L, Xiao H, Yao Y, Qi L, Yao Y (2019) A review on low-dimensional physics-based models of systemic arteries: application to estimation of central aortic pressure. Biomed Eng Online 18(1):41
Acknowledgements
This work was partially supported by the National Key R&D Program of China (Grant No. 2016YFB0200601), the National Natural Science Foundation of China (Grant Nos. 11801543 and 81871447) and the Shenzhen grants (Grant Nos. ZDSYS201703031711426, JCYJ20190806165805433 and SZBL2019062801002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, S., Chen, R., Wu, B. et al. Numerical Simulation of Blood Flows in Patient-specific Abdominal Aorta with Primary Organs. Biomech Model Mechanobiol 20, 909–924 (2021). https://doi.org/10.1007/s10237-021-01419-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01419-7