Abstract
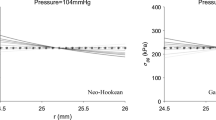
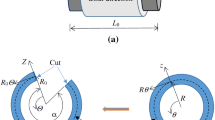
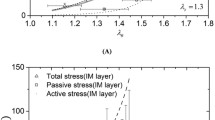
Residual stress is very important for the study of cardiovascular-relevant issues, such as assessing the vulnerability of atherosclerosis and aneurysm. In this paper, the circumferential residual stress of porcine aorta was characterized by combining ex vivo experiments with numerical studies. In the experiments, porcine aortic rings were prepared and cut open, and the cross sections of the opened aortic rings were extracted to construct finite element models. The 5-parameter Mooney–Rivlin model was chosen to describe the tensile mechanical behavior of the aorta. In numerical studies, based on the finite element models and hyperelastic material model, a pulling-back displacement was applied to reclose the models of the opened aortic rings, and the equivalent circumferential residual stress to the pre-opened aorta was analyzed. The results showed that the circumferential residual stress of the aorta generally decreased from the proximal to the distal ends, and the residual stresses of the aortic rings close to the distal end did not show a great difference. This work provides an improved understanding of the residual stress distribution in aorta and may be used as a more realistic initial condition for future stress analysis of the arterial tissue.










Similar content being viewed by others
References
Gasser TC, Ogden RW, Holzapfel GA. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface. 2006;3:15–35.
Jadidi M, Habibnezhad M, Anttila E, Maleckis K, Desyatova A, MacTaggart J, Kamenskiy A. Mechanical and structural changes in human thoracic aortas with age. Acta Biomater. 2020;103:172–88.
García-Herrera CM, Bustosa CA, Celentano DJ, Ortega R. Mechanical analysis of the ring opening test applied to human ascending aortas. Comput Methods Biomech Biomed Eng. 2016;19:1738–48.
Holzapfel GA, Gasser TC, Ogden RW. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast. 2000;61:1–48.
Matsumoto T, Goto T, Furukawa T, Sato M. Residual stress and strain in the lamellar unit of the porcine aorta: experiment and analysis. J Biomech. 2004;37:807–15.
Holzapfel GA, Sommer G, Auer M, Regitnig P, Ogden RW. Layer-specific 3D residual deformations of human aortas with non-atherosclerotic intimal thickening. Ann Biomed Eng. 2007;35:530–45.
Sokolis DP. Effects of aneurysm on the directional, regional, and layer distribution of residual strains in ascending thoracic aorta. J Mech Behav Biomed Mater. 2015;46:229–43.
Fung YC. On the foundations of biomechanics. J Appl Mech Trans ASME. 1983;50:1003–9.
Cieslicki K, Piechna A, Gambin W. Two-layered analytical model of arterial wall with residual stress under physiological loading. Appl Math Model. 2018;57:52–63.
Sokolis DP. Regional distribution of layer-specific circumferential residual deformations and opening angles in the porcine aorta. J Biomech. 2019;96:109335.
Akyildiz AC, Speelman L, van Brummelen H, Gutiérrez MA, Virmani R, van der Lugt A, van der Steen AFW, Wentzel JJ, Gijsen FJH. Effects of intima stiffness and plaque morphology on peak cap stress. Biomed Eng Online. 2011;10:25.
Caille N, Thoumine O, Tardy Y, Meister J-J. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech. 2002;35:177–87.
Ramo NL, Shetye SS, Streijger F, Lee JHT, Troyer KL, Kwon BK, Cripton P, Puttlitz CM. Comparison of in vivo and ex vivo viscoelastic behavior of the spinal cord. Acta Biomater. 2018;68:78–89.
Lee RT, Schoen FJ, Loree HM, Lark MW, Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis: implications for plaque rupture. Arterioscler Thromb Vasc Biol. 1996;16:1070–3.
Ohayon J, Tracqui P. Computation of adherent cell elasticity for critical cell-bead geometry in magnetic twisting experiments. Ann Biomed Eng. 2005;33:131–41.
Barrett SRH, Sutcliffe MPF, Howarth S, Li ZY, Gillard JH. Experimental measurement of the mechanical properties of carotid atherothrombotic plaque fibrous cap. J Biomech. 2009;42:1650–5.
Li ZY, Howarth SPS, Tang T, Graves MJ, U-King-Im J, Trivedi RA, Kirkpatrick PJ, Gillard JH. Structural analysis and magnetic resonance imaging predict plaque vulnerability: a study comparing symptomatic and asymptomatic individuals. J Vasc Surg. 2007;45:768–75.
Li ZY, Howarth S, Trivedi RA, U-King-Im JM, Graves MJ, Brown A, Wang L, Gillard JH. Stress analysis of carotid plaque rupture based on in vivo high resolution MRI. J Biomech. 2006;39:2611–22.
Tang D, Yang C, Mondal S, Liu F, Canton G, Hatsukami TS, Yuan C. A negative correlation between human carotid atherosclerotic plaque progression and plaque wall stress: in vivo MRI-based 2D/3D FSI models. J Biomech. 2008;41:727–36.
Versluis A, Messer HH, Pintado MR. Changes in compaction stress distributions in roots resulting from canal preparation. Int Endod J. 2006;39:931–9.
Cunnane EM, Mulvihill JJE, Barrett HE, Walsh MT. Simulation of human atherosclerotic femoral plaque tissue: the influence of plaque material model on numerical results. Biomed Eng Online. 2015;14:S7.
Lawlor MG, O’Donnell MR, O’Connell BM, Walsh MT. Experimental determination of circumferential properties of fresh carotid artery plaques. J Biomech. 2011;44:1709–15.
Gao H, Long Q. Effects of varied lipid core volume and fibrous cap thickness on stress distribution in carotid arterial plaques. J Biomech. 2008;41:3053–9.
Maher E, Creane A, Sultan S, Hynes N, Lally C, Kelly DJ. Tensile and compressive properties of fresh human carotid atherosclerotic plaques. J Biomech. 2009;42:2760–7.
Chau AH, Chan RC, Shishkov M, Macneill B, Iftimia N, Tearney GJ, Kamm RD, Bouma BE, Kaazempur-Mofrad MR. Mechanical analysis of atherosclerotic plaques based on optical coherence tomography. Ann Biomed Eng. 2004;32:1494–503.
Delfino A, Stergiopulos N, Moore JE Jr, Meister J-J. Residual strain effects on the stress field in a thick wall finite element model of the human carotid bifurcation. J Biomech. 1997;30:777–86.
Mofrad MRK, Younis H, Kaazempur-Mofrad MR, Younis HF, Patel S, Isasi A, Chung C, Chan RC, Hinton DP, Lee RT, Kamm RD. Cyclic strain in human carotid bifurcation and its potential correlation to atherogenesis: idealized and anatomically-realistic models. J Eng Math. 2003;47:299–314.
Teng Z, Zhang Y, Huang Y, Feng J, Yuan J, Lu Q, Sutcliffe MPF, Brown AJ, Jing Z, Gillard JH. Material properties of components in human carotid atherosclerotic plaques: a uniaxial extension study. Acta Biomater. 2014;10:5055–63.
Lally C. Elastic behavior of porcine coronary artery tissue under uniaxial and equibiaxial tension. The effects of cyclic tensile strain and native collagen structure on vascular smooth muscle cells and medial vascular stem cells View project. Artic Ann Biomed Eng. 2004;32:1355–64.
Lillie MA, Shadwick RE, Gosline JM. Mechanical anisotropy of inflated elastic tissue from the pig aorta. J Biomech. 2010;43:2070–8.
Jhun CS, Criscione JC. Characterization of mechanical behavior of a porcine pulmonary artery strip using a randomized uniaxial stretch and stretch-rate protocol. Biomed Eng Online. 2008;7:1–11.
Chen Q, Wang Y, Li ZY. Re-examination of the mechanical anisotropy of porcine thoracic aorta by uniaxial tensile tests. Biomed Eng Online. 2016;15:493–506.
Vaishnav RN, Vossoughi J. Residual stress and strain in aortic segments. J Biomech. 1987;20:235–9.
Laksari K, Shahmirzadi D, Acosta CJ, Konofagou E. Energy-based constitutive modelling of local material properties of canine aortas. R Soc Open Sci. 2016;3:160365.
Sommerville LE, Hartshorne DJ. Intracellular calcium and smooth muscle contraction. Cell Calcium. 1986;7:353–64.
Fung YC, Liu SQ. Change of residual strains in arteries due to hypertrophy caused by aortic constriction. Circ Res. 1989;65:1340–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11772093, 11972118, 61821002) and Australian Research Council (ARC) (Grant No. DP200103492).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Wang, W., Chen, Q. et al. Numerical Determination of the Circumferential Residual Stress of Porcine Aorta by Pulling-Back Method. Acta Mech. Solida Sin. 34, 346–355 (2021). https://doi.org/10.1007/s10338-021-00215-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10338-021-00215-1