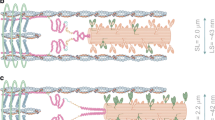
Abstract
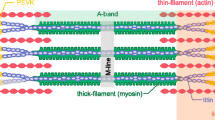
Vertebrate cardiac muscle generates progressively larger systolic force when the end diastolic chamber volume is increased, a property called the “Frank-Starling Law”, or “length dependent activation (LDA)”. In this mechanism a larger force develops when the sarcomere length (SL) increased, and the overlap between thick and thin filament decreases, indicating increased production of force per unit length of the overlap. To account for this phenomenon at the molecular level, we examined several hypotheses: as the muscle length is increased, (1) lattice spacing decreases, (2) Ca2+ sensitivity increases, (3) titin mediated rearrangement of myosin heads to facilitate actomyosin interaction, (4) increased SL activates cross-bridges (CBs) in the super relaxed state, (5) increased series stiffness at longer SL promotes larger elementary force/CB to account for LDA, and (6) stretch activation (SA) observed in insect muscles and LDA in vertebrate muscles may have similar mechanisms. SA is also known as delayed tension or oscillatory work, and universally observed among insect flight muscles, as well as in vertebrate skeletal and cardiac muscles. The sarcomere stiffness observed in relaxed muscles may significantly contributes to the mechanisms of LDA. In vertebrate striated muscles, the sarcomere stiffness is mainly caused by titin, a single filamentary protein spanning from Z-line to M-line and tightly associated with the myosin thick filament. In insect flight muscles, kettin connects Z-line and the thick filament to stabilize the sarcomere structure. In vertebrate cardiac muscles, titin plays a similar role, and may account for LDA and may constitute a molecular mechanism of Frank-Starling response.



Modified from Fig. 3 of (Davis et al. 2002), and reproduced with permission from Elsevier




Similar content being viewed by others
Availability of data and materials
The data are available by the authors on reasonable request.
References
Abbott RH (1972) An interpretation of the effects of fiber length and calcium on the mechanical properties of insect flight muscle. Cold Spring Hbr Symp on Quant Biol 37:647–654
Abbott RH, Steiger GJ (1977) Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol 266:13–42
Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, de Tombe PP (2016) Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci USA 113:2306–2311
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415:198–205
Bullard B, Garcia T, Benes V, Leake MV, Linke WA, Oberhauser AF (2006) The molecular elasticity of the insect flight muscle proteins projectin and kettin. Proc Natl Acad Sci USA 103:4451–4456
Bullard B, Goulding D, Ferguson C, Leonard K (2000) Links in the chain: the contribution of kettin to the elasticity of insect muscles. Adv Exp Med Biol 481:207–218; discussion 219–220
Bullard B, Linke WA, Leonard K (2002) Varieties of elastic protein in invertebrate muscles. J Muscle Res Cell Motil 23:435–447
Bullard B, Pastore A (2019) Through thick and thin: dual regulation of insect flight muscle and cardiac muscle compared. J Muscle Res Cell Motil 40:99–110
Davis JS, Satorius CL, Epstein ND (2002) Kinetic effects of myosin regulatory light chain phosphorylation on skeletal muscle contraction. Biophys J 83:359–370
de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC (2010) Myofilament length dependent activation. J Mol Cell Cardiol 48:851–858
Ebashi S, Endo M (1968) Calcium ion and muscle contraction. Prog Biophys Mol Biol 18:123–183
Evans DJ, Searles DJ, Mittag E (2001) Fluctuation theorem for Hamiltonian systems—Le Chatelier’s principle. Phys Rev E. https://doi.org/10.1103/PhysRevE.63.051105
Fabiato A, Fabiato F (1978) Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol 72:667–699
Farman GP, Allen EJ, Schoenfelt KQ, Backx PH, de Tombe pp. (2010) The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys J 99:2978–2986
Farman GP, Gore D, Allen E, Schoenfelt K, Irving TC, de Tombe pp. (2011) Myosin head orientation: a structural determinant for the Frank-Starling relationship. Am J Physiol Heart Circ Physiol 300:H2155-2160
Fujita H, Labeit D, Gerull B, Labeit S, Granzier HL (2004) Titin isoform-dependent effect of calcium on passive myocardial tension. Am J Physiol Heart Circ Physiol 287:H2528-2534
Fukuda N, Granzier HL, Ishiwata S, Kurihara S (2008) Physiological functions of the giant elastic protein titin in mammalian striated muscle. J Physiol Sci 58:151–159
Fukuda N, Sasaki D, Ishiwata S, Kurihara S (2001) Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circulation 104:1639–1645
Fukuda N, Wu Y, Nair P, Granzier HL (2005) Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol 125:257–271
Galler S, Wang BG, Kawai M (2005) Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J 89:3248–3260
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192
Heinl P, Kuhn HJ, Ruegg JC (1974) Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol 237:243–258
Huang QQ, Brozovich FV, Jin JP (1999) Fast skeletal muscle troponin T increases the cooperativity of transgenic mouse cardiac muscle contraction. J Physiol 520(Pt 1):231–242
Huxley AF (1974) Muscular contraction. J Physiol 243:1–43
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Irving M (2017) Regulation of contraction by the thick filaments in skeletal muscle. Biophys J 113:2579–2594
Iwamoto H (2011) Structure, function and evolution of insect flight muscle. Biophysics (Nagoya-shi) 7:21–28
Jin JP, Zhang Z, Bautista JA (2008) Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr 18:93–124
Josephson RK, Malamud JG, Stokes DR (2000) Asynchronous muscle: a primer. J Exp Biol 203:2713–2722
Kawai M (1978) Head rotation or dissociation? A study of exponential rate processes in chemically skinned rabbit muscle fibers when MgATP concentration is changed. Biophys J 22:97–103
Kawai M (1986) The role of orthophosphate in crossbridge kinetics in chemically skinned rabbit psoas fibres as detected with sinusoidal and step length alterations. J Muscle Res Cell Motil 7:421–434
Kawai M (2018) Mathematics needed to solve problems of contraction. Biomechanics, muscle fibers, and how to interface experimental apparatus to a computer (textbook). Springer International Publishing AG, London, pp 65–76
Kawai M, Brandt P, Orentlicher M (1977) Dependence of energy transduction in intact skeletal muscles on the time in tension. Biophys J 18:161–172
Kawai M, Brandt PW (1980) Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Mot 1:279–303
Kawai M, Halvorson H (1989) Role of MgATP and MgADP in the crossbridge kinetics in chemically skinned rabbit psoas fibers. Study of a fast exponential process C. Biophys J 55:595–603
Kawai M, Halvorson HR (1991) Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas. Biophys J 59:329–342
Kawai M, Halvorson HR (2007) Force transients and minimum cross-bridge models i muscular contraction. J Muscle Res Cell Motil 28:371–395
Kawai M, Karam TS, Kolb J, Wang L, Granzier HL (2018) Nebulin increases thin filament stiffness and force per cross-bridge in slow-twitch soleus muscle fibers. J Gen Physiol 150:1510–1522
Kazmierczak K, Xu Y, Jones M, Guzman G, Hernandez OM, Kerrick WG, Szczesna-Cordary D (2009) The role of the N-terminus of the myosin essential light chain in cardiac muscle contraction. J Mol Biol 387:706–725
Kobirumaki-Shimozawa F, Inoue T, Shintani SA, Oyama K, Terui T, Minamisawa S, Ishiwata S, Fukuda N (2014) Cardiac thin filament regulation and the Frank-Starling mechanism. J Physiol Sci 64:221–232
Kolb J, Li F, Methawasin M, Adler M, Escobar YN, Nedrud J, Pappas CT, Harris SP, Granzier H (2016) Thin filament length in the cardiac sarcomere varies with sarcomere length but is independent of titin and nebulin. J Mol Cell Cardiol 97:286–294
Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP (2003) Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547:951–961
Le Chatelier H, Boudouard O (1898) Limits of flammability of gaseous mixtures. Bull Soc Chim France (Paris) 19:483–488
Li H, Linke WA, Oberhauser AF, Carrion-Vazquez M, Kerkvliet JG, Lu H, Marszalek PE, Fernandez JM (2002) Reverse engineering of the giant muscle protein titin. Nature 418:998–1002
Littlefield R, Fowler VM (2002) Measurement of thin filament lengths by distributed deconvolution analysis of fluorescence images. Biophys J 82:2548–2564
Lowey S, Saraswat LD, Liu H, Volkmann N, Hanein D (2007) Evidence for an interaction between the SH3 domain and the N-terminal extension of the essential light chain in class II myosins. J Mol Biol 371:902–913
Lu X, Bryant MK, Bryan KE, Rubenstein PA, Kawai M (2005) Role of the N-terminal negative charges of actin in force generation and cross-bridge kinetics in reconstituted bovine cardiac muscle fibres. J Physiol 564:65–82
Lu X, Tobacman LS, Kawai M (2006) Temperature-dependence of isometric tension and cross-bridge kinetics of cardiac muscle fibers reconstituted with a tropomyosin internal deletion mutant. Biophys J 91:4230–4240
Maruyama K, Kimura S, Ohashi K, Kuwano Y (1981) Connectin, an elastic protein of muscle. Identification of “titin” with connectin. J Biochem 89:701–709
Maruyama K, Natori R, Nonomura Y (1976) New elastic protein from muscle. Nature 262:58–60
Michael JJ, Gollapudi SK, Ford SJ, Kazmierczak K, Szczesna-Cordary D, Chandra M (2013) Deletion of 1–43 amino acids in cardiac myosin essential light chain blunts length dependency of Ca(2+) sensitivity and cross-bridge detachment kinetics. Am J Physiol Heart Circ Physiol 304:H253-259
Mijailovich SM, Stojanovic B, Nedic D, Svicevic M, Geeves MA, Irving TC, Granzier HL (2019) Nebulin and titin modulate cross-bridge cycling and length-dependent calcium sensitivity. J Gen Physiol 151:680–704
Millman BM (1998) The filament lattice of striated muscle. Physiol Rev 78:359–391
Muir WW, Hamlin RL (2020) Myocardial contractility: historical and contemporary considerations. Front Physiol 11:222
Muthu P, Wang L, Yuan CC, Kazmierczak K, Huang W, Hernandez OM, Kawai M, Irving TC, Szczesna-Cordary D (2011) Structural and functional aspects of the myosin essential light chain in cardiac muscle contraction. FASEB J 25:4394–4405
Nocella M, Colombini B, Bagni MA, Bruton J, Cecchi G (2012) Non-crossbridge calcium-dependent stiffness in slow and fast skeletal fibres from mouse muscle. J Muscle Res Cell Motil 32:403–409
Ohtsuki I (1979) Number of anti-troponin striations along the thin filament of chick embryonic breast muscle. J Biochem 85:1377–1378
Page SG, Huxley HE (1963) Filament lengths in striated muscle. J Cell Biol 19:369–390
Perz-Edwards RJ, Irving TC, Baumann BA, Gore D, Hutchinson DC, Kržič U, Porter RL, Ward AB, Reedy MK (2011) X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc Natl Acad Sci USA 108:120–125
Piazzesi G, Caremani M, Linari M, Reconditi M, Lombardi V (2018) Thick filament mechano-sensing in skeletal and cardiac muscles: a common mechanism able to adapt the energetic cost of the contraction to the task. Front Physiol 9:736
Pringle JW (1967) The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol 17:1–60
Reconditi M, Caremani M, Pinzauti F, Powers JD, Narayanan T, Stienen GJ, Linari M, Lombardi V, Piazzesi G (2017) Myosin filament activation in the heart is tuned to the mechanical task. Proc Natl Acad Sci USA 114:3240–3245
Ruegg JC, Tregear RT (1966) Mechanical factors affecting the ATPase activity of glycerol-extracted insect fibrillar flight muscle. Proc R Soc Lond B Biol Sci 165:497–512
Sheng JJ, Jin JP (2016) TNNI1, TNNI2 and TNNI3: Evolution, regulation, and protein structure-function relationships. Gene 576:385–394
Shiels HA, White E (2008) The Frank-Starling mechanism in vertebrate cardiac myocytes. J Exp Biol 211:2005–2013
Smith DA (1998) A strain-dependent ratchet model for [phosphate]- and [ATP]-dependent muscle contraction. J Muscle Res Cell Motil 19:189–211
Smith DA, Geeves MA (1995) Strain-dependent cross-bridge cycle for muscle. Biophys J 69:524–537
Stelzer JE, Moss RL (2006) Contributions of stretch activation to length-dependent contraction in murine myocardium. J Gen Physiol 128:461–471
Tawada K, Kawai M (1990) Covalent cross-linking of single fibers from rabbit psoas increases oscillatory power. Biophys J 57:643–647
Terui T, Shimamoto Y, Yamane M, Kobirumaki F, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N (2010) Regulatory mechanism of length-dependent activation in skinned porcine ventricular muscle: role of thin filament cooperative activation in the Frank-Starling relation. J Gen Physiol 136:469–482
Tonino P, Kiss B, Strom J, Methawasin M, Smith JE 3rd, Kolb J, Labeit S, Granzier H (2017) The giant protein titin regulates the length of the striated muscle thick filament. Nat Commun 8:1041
Trayer IP, Trayer HR, Levine BA (1987) Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur J Biochem 164:259–266
Vale RD, Oosawa F (1990) Protein motors and Maxwell’s demons: does mechanochemical transduction involve a thermal ratchet? Adv Biophys 26:97–134
Wang G, Kawai M (1996) Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibers. Biophys J 71:1450–1461
Wang G, Kawai M (1997) Force generation and phosphate release steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys J 73:878–894
Wang L, Kawai M (2013) A re-interpretation of the rate of tension redevelopment (kTR) in active muscle. J Muscle Res Cell Motil 34:407–415
Wang K, McClure J, Tu A (1979) Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci 76(8):3698–3702
Wang Q, Newhard CS, Ramanath S, Sheppard D, Swank DM (2014) An embryonic myosin converter domain influences Drosophila indirect flight muscle stretch activation, power generation and flight. J Exp Biol 217:290–298
Wei B, Jin JP (2016) TNNT1, TNNT2, and TNNT3: isoform genes, regulation, and structure-function relationships. Gene 582:1–13
Whitten AE, Jeffries CM, Harris SP, Trewhella J (2008) Cardiac myosin-binding protein C decorates F-actin: implications for cardiac function. Proc Natl Acad Sci USA 105:18360–18365
Wijnker PJ, Sequeira V, Foster DB, Li Y, Dos Remedios CG, Murphy AM, Stienen GJ, van der Velden J (2014) Length-dependent activation is modulated by cardiac troponin I bisphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol Heart Circ Physiol 306:H1171-1181
Wu S, Liu J, Reedy MC, Tregear RT, Winkler H, Franzini-Armstrong C, Sasaki H, Lucaveche C, Goldman YE, Reedy MK, Taylor KA (2010) Electron tomography of cryofixed, isometrically contracting insect flight muscle reveals novel actin-myosin interactions. PLoS ONE 5:e12643
Zhang Z, Akhter S, Mottl S, Jin JP (2011) Calcium-regulated conformational change in the C-terminal end segment of troponin I and its binding to tropomyosin. Febs J 278:3348–3359
Zhao Y, Kawai M (1993) The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J 64:197–210
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (HL127691, HL138007 and HL146676 to J.-P.J).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawai, M., Jin, JP. Mechanisms of Frank-Starling law of the heart and stretch activation in striated muscles may have a common molecular origin. J Muscle Res Cell Motil 42, 355–366 (2021). https://doi.org/10.1007/s10974-020-09595-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-020-09595-2