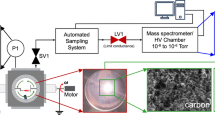
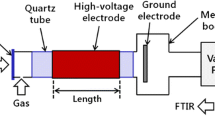
Abstract
A comprehensive study of the reversed arc plasma enhanced CVD (RACVD) reactor utilizing an Ar + H2 + CH4 plasma-creating mixture in the pressure range 1–100 Torr with a plasma flow direction opposite to the direction of the arc current was carried out. The reversed arc discharge has rising current–voltage characteristics showing voltage increasing with pressure and hydrogen concentration. The spectrum of the Ar-H2-CH4 plasma column includes CH, C2, and H2 molecular bands, in addition to Hα, Hβ, Hγ, and Hδ lines. The dissociation degree of H2 was estimated from the intensity ratio IHα/IArI of the Hα and ArI 750 nm lines using the optical actinometry method, yielding an average dissociation degree of hydrogen in the arc plasma of 15–20%. The average vibrational and rotational temperatures of CH radicals are Tv = Tr = 3000 K ± 300 K. The dissociation degree of hydrogen in the reversed arc discharge was calculated by the advection–diffusion-reaction model and showed reasonably good agreement both with experimental findings and with LTE calculations. The high concentration of nascent hydrogen and hydrocarbon radicals in the reversed arc plasma and its uniform distribution across the arc column makes it suitable for diamond coatings. The results obtained on the interaction of reversed arc plasma with substrates suspended within the current-carrier arc plasma column were applied to the description of a dusty reversed arc plasma in fluidized bed reactors. It was found that the energy effectiveness of the treatment of nanoparticles in the RACVD fluidized bed reactor exceeds 90%.





















Similar content being viewed by others
References
Qing Z, Otorbaev DK, Brussaard GJH, Van de Sanden MCM, Schram DC (1996) Diagnostics of the magnetized low-pressure hydrogen plasma jet: molecular regime. J Appl Phys 80:1312
Hruby V et al (1997) Methane arc jet development. IEPC-97–014, 104–111
Starikovskiy A et al (2012) Ignition of hydrocarbon–air mixtures with non-equilibrium plasma at elevated pressures. 50th AIAA Aerospace Sciences Meeting, Nashville, Tennessee
Cho W et al (2004) Conversion of natural gas to hydrogen and carbon black by plasma and application of plasma black. Prepr Pap Am Chem Soc Div Fuel Chem 49(1):181
Choi S et al (2006) Continuous process of carbon nanotubes synthesis by decomposition of methane using an arc-jet plasma. Thin Solid Films 506:244
Diamond Films ’94 (1994). Bachmann PK, Buckley-Golder IM, Glass JT, and Kamo M (eds) Elsevier, Amsterdam
Gorokhovsky V (2019) Reactors for plasma-assisted processes and associated methods. US Pat No 10304665
Gorokhovsky V (2021) Characterization of reversed arc hydrocarbon plasma in material synthesis. In: 67th AVS international symposium, Paper No 56553, 2021 October 24–29, Charlotte, NC
Avtaeva S, Gorokhovsky V, Obrusnik A, Zembower Z (2017) Composition of high current arc plasma in Ar-H2 mixture at moderate pressures. In: XIII international conference on gas-discharge plasma and its applications, September 2017, Novosibirsk, Russia
Avtaeva S (2019) H2 dissociation in Ar–H2 arc discharge of moderate pressure. Plasma Res Express 1:015018
Lavrov BP, Melnikov AS, Käning M, Röpcke J (1999) UV continuum emission and diagnostics of hydrogen-containing nonequilibrium plasmas. Phys Rev E 59:3526
Avtaeva SV, Lapochkina TM (2007) Characteristics of molecular hydrogen and CH* radicals in a methane plasma in a magnetically enhanced capacitive RF discharge. Plasma Phys Rep 33:774–785
Gorokhovsky V (2005) Characterization of cascade arc assisted CVD diamond coating technology, Part I. Plasma processing parameters. Surf Coat Tech 194:344–362
Gorokhovsky V (2005) Characterization of cascade arc assisted CVD diamond coating technology, Part II. Coating properties and applications. Surf Coat Technol 194:300–318
Avtaeva S, Gorokhovsky V, Robertson S, Shunko E (2020) Characterization of low-pressure arc plasma in large volumes. Specrochimica Acta Part B 165:105785
Asinovsky EI, Kirillin AV, Nizovsky VL (1991) Stabilized electric arcs and their applications in thermophysical experiment. Nauka, Moscow, p 264
Boulos M, Fauchais P, Pfender E (1994) Thermal plasmas. Fundamentals and applications. Plenum Press, New York
Pastol A, Catherine Y (1990) Optical emission spectroscopy for diagnostic and monitoring of CH4 plasmas used for α-C: H deposition. J Phys D 23:799
Reeve SW, Weimer WA, Cerioa FM (1993) Gas phase chemistry in a direct current plasma jet diamond reactor. J Appl Phys 74:7521–7530
Juchmann W, Luque J, Wolfrum J, Jeffries JB (1998) Absolute concentration, temperature, and velocity measurements in a diamond depositing dc-arcjet reactor. Diam Relat Mater 7:165–169
Mankelevich YuA, Suetin NV, Ashfold MNR, Boxford WE, Orr-Ewing AJ, Smith JA, Willsb JB (2003) Chemical kinetics in carbon depositing d.c.-arc jet CVD reactors. Diam Relat Mater 12:383–390
Herrebout D, Bogaerts A, Yan M, Gijbels R, Goedheer W, Vanhulsel A (2002) Modeling of a capacitively coupled radio-frequency methane plasma: comparison between a one-dimensional and a two-dimensional fluid model. J Appl Phys 92:2290–2295
Beulens JJ (1992) Surface modification using a cascade arc source. PhD Thesis, Eindhoven University of Technology, Eindhoven
Van de Graaf MJ (1994) A new hydrogen particle source. PhD Thesis, Technical University Eindhoven, Eindhoven
Radtsig AA and Smirnov BM (1978) Reference data on atoms, molecules, and ions. Moscow: Atomizdat; (1985) Springer, Berlin
Avtaeva SV, Mamytbekov MZ, Otorbaev DK (1997) Diagnostics of magnetically enhanced RF discharges in methane, argon and methane-argon mixtures. J Phys D Appl Phys 30:3000–3007
Galtsev VE, Ivanov YuA, Slovetskii DI, Rytova NM, Timakin VN (1983) Mechanism of excitation of Ar and H atoms and the atomic hydrogen density in the positive column of a glow discharge in Ar + CH4 mixtures. High Energy Chem 17(2):164 ((in Russian))
Gruzdev PF (1990) Transition probabilities and radiative lifetimes of atomic and ionic levels. Énergoatomizdat, Moscow
Calloway J (1988) Electron-impact excitation of hydrogen atoms: Energies between the n=3 and n=4 threshold. Phys Rev A 37:3692
Chutjon A, Cartwright DC (1981) Electron-impact excitation of electronic states in argon at incident energies between 16 and 100 eV. Phys Rev A 23:2178
Möhlmann GR, de Heer FJ (1976) Emission cross sections of the H2 (3p3Πu→2s3Σ+g transition) for electron impact on H2. Chem Phys Lett 42:240–244
Gorokhovsky V, Del Belluz P (2013) Ion treatment by low pressure arc plasma immersion surface engineering processes. Surf Coat Technol 215:431–439
Gorokhovsky V (2012) Modeling of DC discharges in argon at low pressures. In: Presentation at the 12th COMSOL conference, Boston
COMSOL (2018) Plasma module user’s guide
Murphy AB (2000) Transport coefficients of hydrogen and argon–hydrogen plasmas. Plasma Chem Plasma Proc 20:279–297
Barreto PRP, Bosco E Del (1994) Characterization of a plasma jet produced by a vacuum discharge for diamond deposition. In: Proceedings of the international conference on plasma physics, Brazil, pp 261–264
Gerasimov, GYa, Shatalov OP (2013) Kinetic mechanism of combustion of hydrogen–oxygen mixtures. Journal of Engineering Physics and Thermophysics 86:987–995
Vatolin NA, Moiseev GK, Trusov BG (1994) Thermodynamic modeling of high temperature inorganic systems. Metallurgy, Moscow
Gorokhovsky V et al (2006) Tribological performance of hybrid filtered arc-magnetron coatings Part I: Coating deposition process and basic coating properties characterization. Surf Coat Technol 201:3732–3747
Snail KA, Marks CM (1992) In situ diamond growth rate measurement using emission interferometry. Appl Phys Lett 60:3135
Hanssen LM, Snail KA, Carrington WA, Butler JE, Kellogg S, Oakes DB (1991) Diamond and non-diamond carbon synthesis in an oxygen-acetylene flame. Thin Solid Films 196:271–281
Snail K, Craigie C (1991) Synthesis of high quality diamond films in a turbulent flame. Appl Phys Lett 58:1875–1877
Incropera F, De Witt D (1985) Fundamentals of heat and mass transfer. Wiley, New York
Ravindra NM et al (1998) Temperature-dependent emissivity of silicon-related materials and structures. IEEE Trans Semicond Manuf 11:30–38
Maurer HR, Kersten H (2011) On the heating of nano- and microparticles in process plasmas. J Phys D Appl Phys 44:174029
Pekker L, Hussary N (2014) Effect of boundary conditions on the heat flux to the wall in two-temperature modeling of “thermal” plasmas. J Phys D Appl Phys 47:445202
Lee YC, Chyou YP, Pfender E (1985) Particle dynamics and particle heat and mass transfer in thermal plasmas. Part II. Particle heat and mass transfer in thermal plasmas. Plasma Chem Plasma Proc 5:391–414
Chen X, Pfender E (1983) Effect of the Knudsen number on heat transfer to a particle immersed into a thermal plasma. Plasma Chem Plasma Proc 3:97
Acknowledgments
The authors are very grateful to J. Williams, T. Munsat and J. Trelles for useful comments and suggestions, to V. M. Donnelly and Yi-Kang Pu for discussions on OES plasma diagnostics, to D. Smith and A. Obrusnik for consultations on transport properties settings of the COMSOL model of the arc discharge, and to Z. Zembower and O. Popov, for their support with the experimental work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avtaeva, S., Gorokhovsky, V. Characterization of Reversed Arc Hydrocarbon Plasma in Material Processing. Plasma Chem Plasma Process 41, 815–839 (2021). https://doi.org/10.1007/s11090-021-10153-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10153-y