Abstract
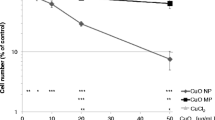
We previously described a non-monotonic dose response curve at low copper concentrations where 3.125 μM CuSO4 (the early inflection point) was more toxic than 25 μM CuSO4 in Caco-2 cells. We employed global proteomics to investigate this observation. The altered expression levels of a small number of proteins displaying a temporal response may provide the best indication of the underlying mechanism; more well-known copper response proteins including the metal binding metallothioneins (MT1X, MT1F, MT2A) and antioxidant response proteins including Heme oxygenase were upregulated to a similar level in both copper concentrations and so are less likely to underpin this phenomenon.
The temporal response proteins include Granulins, AN1-type zinc finger protein 2A (ZFAND2A), and the heat shock proteins (HSPA6 and HSPA1B). Granulins were decreased after 4 h only in 25 μM CuSO4 but from 24 h, were decreased in both copper concentrations to a similar level. Induction of ZFAND2A and increases in HSPA6 and HSPA1B were observed at 24 h only in 25 μM CuSO4 but were present at 48 h in both copper conditions. The early expression of ZFAND2A, HSPs, and higher levels of α-crystallin B (CRYAB) correlated with lower levels of misfolded proteins in 25 μM CuSO4 compared to 3.125 μM CuSO4 at 48 h. These results suggest that 3.125 μM CuSO4 at early time points was unable to activate the plethora of stress responses invoked by the higher copper concentration, paradoxically exposing the Caco-2 cells to higher levels of misfolded proteins and greater proteotoxic stress.





Similar content being viewed by others
References
Allocca S, Ciano M, Ciardulli MC, D’Ambrosio C, Scaloni A, Sarnataro D, Caporaso MG, D’Agostino M, Bonatti S (2018) An αB-crystallin peptide rescues compartmentalization and trafficking response to Cu overload of ATP7B-H1069Q, the most frequent cause of Wilson disease in the Caucasian population. Int J Mol Sci. 19(7):1892
Araya M, Pixarro F, Olivares M, Arredondo M, Gonzálex M, Méndez M (2006) Understanding copper homeostasis in humans and copper effects on health. Biol Res 39:183–187
Baker M, Mackenzie I, Pickering-Brown S, Gass J, Rademakers R, Lindholm C, SnowdenJ AJ, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Beele S, Moisse M, Damme M, De Muynck L, Robberecht W, Van Den Bosch L, Saftig P, Van Damme P (2017) Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Human Mol Gen 26(15):2850–2863
Bremner I (1998) Manifestations of copper excess, The American Journal of Clinical Nutrition. Volume 67(5):1069S–1073S
Calabrese EJ (2013) Hormetic mechanisms. Crit Rev Toxicol 43(7):580–606
Coleman O, Henry M, Clynes M, Meleady P (2017) Filter-aided sample preparation (FASP) for improved proteome analysis of recombinant chinese hamster ovary cells. In: Meleady P. (eds) Heterologous protein production in CHO cells. Methods in Molecular Biology, vol 1603. Humana Press, New York, NY
Coleman O, Henry M, O’Neill F, Roche S, Swan N, Boyle L, Murphy J, Meiller J, Conlon NT, Geoghegan J, Conlon KC, Lynch V, Straubinger NL, Straubinger RM, McVey G, Moriarty M, Meleady P, Clynes M (2018) A comparative quantitative LC-MS/MS profiling analysis of human pancreatic adenocarcinoma, adjacent-normal tissue, and patient-derived tumour xenografts. Proteomes 6(4):45
Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD (2006) A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res 66(13):6800–6806
Freedman JH, Weiner RJ, Peisach J (1986) Resistance to copper toxicity of cultured hepatoma cells. Characterization of resistant cell lines. J Biol Chem. 261(25):11840–11848
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. JBC 143(7):1883–1898
Kardos J, Héja L, Simon Á, Jablonkai I, Kovács R, Jemnitz K (2018) Copper signalling: causes and consequences. Cell Commun Signal 16(1):80
Karginova O, Weekley CM, Raoul A, Alsayed A, Wu T, Lee SSY, He C, Olopade OI (2019) Inhibition of copper transport induces apoptosis in triple-negative breast cancer cells and suppresses tumor angiogenesis. Mol Cancer Ther. 18(5):873–885
Klein ZA, Takahashi H, Ma M, Stagi M, Zhou M, Lam TT, Strittmatter SM (2017) Loss of TMEM106B ameliorates lysosomal and frontotemporal dementia-related phenotypes in progranulin-deficient mice. Neuron. 95(2):281–296
Lee SH, Lee S, Du J, Jain K, Ding M, Kadado AJ, Atteya G, Jagi Z, Tyagi T, Kim W, Herzog RI, Patel A, Ionescu CN, Martin KA, Hwa J (2019) Mitochondrial MsrB2 serves as a switch and transducer for mitophagy. EMBO Mol Med. 11(8):e10409
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. The American Journal of Clinical Nutrition 63(5):797S–811S
McConkey DJ (2017) The integrated stress response and proteotoxicity in cancer therapy. Biochem Biophys Res Commun. 482(3):450–453
McElwee MK, Song MO, Freedman JH (2009) Copper activation of NF-kappaB signaling in HepG2 cells. J Mol Biol. 393(5):1013–1021.
Mizzen CA, Cartel NJ, Yu WH, Fraser PE, McLachlan DR (1996) Sensitive detection of metallothioneins-1, -2 and -3 in tissue homogenates by immunoblotting: a method for enhanced membrane transfer and retention. J Biochem Biophys Met 32(2):77–83
Murphy S, Zweyer M, Raucamp M, Henry M, Meleady P, Swandulla D, Ohlendieck K (2019) Proteomic profiling of the mouse diaphragm and refined mass spectrometric analysis of the dystrophic phenotype. J Muscle Res Cell Motil. 40(1):9–28
Nevitt T, Ohrvik H, Thiele DJ (2012) Charting the travels of copper in eukaryotes from yeast to mammals. BBA 1823(9):1580–1593
Nivon M, Fort L, Muller P, Richet E, Simon S, Guey B, Fournier M, Arrigo AP, Hetz C, Atkin JD, Kretz-Remy C (2016) NFκB is a central regulator of protein quality control in response to protein aggregation stresses via autophagy modulation. Mol Biol Cell. 27(11):1712–1727
O’Doherty C, Keenan J, Horgan K, Murphy R, O’Sullivan F, Clynes M (2019) Copper-induced non-monotonic dose response in Caco-2 cells. In Vitro Cellular & Developmental Biology - Animal. https://doi.org/10.1007/s11626-019-00333-8
Oliveros JC (2007-2015). Venny. an interactive tool for comparing lists with Venn’s diagram. Available from: http://bioinfogp.cnb.csic.es/ tools/venny/index.html
Paushter DH, Du H, Feng T, Hu F (2018) The lysosomal function of progranulin, a guardian against neurodegeneration. Acta Neuropathol. 36(1):1–17
Polishchuk EV, Concilli M, Iacobacci S, Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D, van Ijzendoorn SC, Chan J, Cheng CJ, Amoresano A, Pane F, Pucci P, Tarallo A, Parenti G, Brunetti-Pierri N, Settembre C, Ballabio A, Polishchuk RS (2014) Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev Cell. 29(6):686–700
Rossi A, Riccio A, Coccia M, Trotta E, La Frazia S, Santoro MG (2014) The proteasome inhibitor bortezomib is a potent inducer of zinc finger AN1-type domain 2a gene expression: role of heat shock factor 1 (HSF1)-heat shock factor 2 (HSF2) heterocomplexes. J Biol Chem. 289(18):12705–12715
Rossi A, Trotta E, Brandi R, Arisi I, Coccia M, Santoro MG (2010) AIRAP, a new human heat shock gene regulated by heat shock factor 1. J Biol Chem. 285(18):13607–13615
Schulz H (1888) Ueber Hefegifte. Pflügers Arch 42(1):517–541
Sok J, Calfon M, Lu J, Lichtlen P, Clark SG, Ron D (2001) Arsenite-inducible RNA-associated protein (AIRAP) protects cells from arsenite toxicity. Cell Stress Chaperones 6(1):6–15
Tanaka Y, Suzuki G, Matsuwaki T, Hosokawa M, Serrano G, Beach TG, Yamanouchi K, Hasegawa M, Nishihara M (2017) Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Human Molecular Genetics. 26(5):969–988
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455
Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, Jiang H (2012) Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Digestive Diseases and Sciences 57(7):1792–1801
Worsøe J, Fynne L, Gregersen T, Schlageter V, Christensen LA, Dahlerup JF, Rijkhoff NJM, Laurberg S, Krogh K (2011) Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterology 11(1):145
Yang WC, Tsai WC, Lin PM, Yang MY, Lin YC, Chang CS, Yu WH, Lin SF (2013) Human BDH2, an anti-apoptosis factor, is a novel poor prognostic factor for de novo cytogenetically normal acute myeloid leukemia. J Biomed Sci. 20(1):58.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Shouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1-2):317–331
Zhou X, Sun L, Brady OA, Murphy KA, Hu F (2017) Elevated TMEM106B levels exaggerate lipofuscin accumulation and lysosomal dysfunction in aged mice with progranulin deficiency. Acta Neuropathol Commun. 5(1):9
Yang-Yen HF (2006) Mcl-1: a highly regulated cell death and survival controller. J. Biomed. Sci. 13(2):201–204
Funding
This work was funded by a joint Enterprise Ireland Innovative Partnership programme (IP/2015/0375).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
JK and PM are joint first authors.
RM and FOS are joint last authors.
Rights and permissions
About this article
Cite this article
Keenan, J., Meleady, P., O’Doherty, C. et al. Copper toxicity of inflection point in human intestinal cell line Caco-2 dissected: influence of temporal expression patterns. In Vitro Cell.Dev.Biol.-Animal 57, 359–371 (2021). https://doi.org/10.1007/s11626-020-00540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00540-8