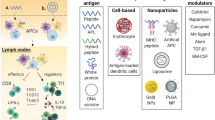
Abstract
Purpose of Review
Type 1 diabetes (T1D) can be managed by insulin replacement, but it is still associated with an increased risk of microvascular/cardiovascular complications. There is considerable interest in antigen-specific approaches for treating T1D due to their potential for a favorable risk-benefit ratio relative to non-specific immune-based treatments. Here we review recent antigen-specific tolerance approaches using auto-antigen and/or immunomodulatory agents in NOD mice and provide insight into seemingly contradictory findings.
Recent Findings
Although delivery of auto-antigen alone can prevent T1D in NOD mice, this approach may be prone to inconsistent results and has not demonstrated an ability to reverse established T1D. Conversely, several approaches that promote presentation of auto-antigen in a tolerogenic context through cell/tissue targeting, delivery system properties, or the delivery of immunomodulatory agents have had success in reversing recent-onset T1D in NOD mice.
Summary
While initial auto-antigen based approaches were unable to substantially influence T1D progression clinically, recent antigen-specific approaches have promising potential.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Salisbury EM, Game DS, Lechler RI. Transplantation tolerance. Pediatr Nephrol. Springer Verlag. 2014;29:2263–72. https://doi.org/10.1007/s00467-013-2659-5.
Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M. The challenge of modulating β-cell autoimmunity in type 1 diabetes [Internet]. Lancet Diabetes Endocrinol. Lancet Publishing Group. 2019;7:52–64. https://doi.org/10.1016/S2213-8587(18)30112-8.
Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. https://doi.org/10.1084/jem.20061631.
Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. Frontiers Media S.A. 2019;10:43.
Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective Interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97.
Abbas AK, Trotta E, Simeonov DR, Marson A, Bluestone JA. Revisiting IL-2: Biology and therapeutic prospects. Sci Immunol. American Association for the Advancement of Science. 2018;3:eaat1482. https://doi.org/10.1126/sciimmunol.aat1482.
Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care Diabetes Care. 2011;34:2026–32. https://doi.org/10.2337/dc11-0472.
Phillips BE, Garciafigueroa Y, Engman C, Trucco M, Giannoukakis N. Tolerogenic dendritic cells and T-regulatory cells at the clinical trials crossroad for the treatment of autoimmune disease; emphasis on type 1 diabetes therapy [Internet]. Front Immunol. Frontiers Media S.A. 2019;10:148. https://doi.org/10.3389/fimmu.2019.00148.
Zhang L, Crawford F, Yu L, Michels A, Nakayama M, Davidson HW, et al. Monoclonal antibody blocking the recognition of an insulin peptide-MHC complex modulates type 1 diabetes. Proc Natl Acad Sci U S A. National Academy of Sciences. 2014;111:2656–61. https://doi.org/10.1073/pnas.1323436111.
Ostrov DA, Alkanani A, McDaniel KA, Case S, Baschal EE, Pyle L, et al. Methyldopa blocks MHC class II binding to disease-specific antigens in autoimmune diabetes. J Clin InvestAmerican Society for Clinical Investigation. 2018;128:1888–902. https://doi.org/10.1172/JCI97739.
Serra P, Santamaria P. Antigen-specific therapeutic approaches for autoimmunity [Internet]. Nat Biotechnol. 2019;37:238–51. https://doi.org/10.1038/s41587-019-0015-4.
Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64.
Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work [Internet]. Nat Rev Immunol. Nature Publishing Group. 2008;8:523–32. https://doi.org/10.1038/nri2343.
Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. Nature Publishing Group. 2010;11:1093–101.
Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. The American Society for Clinical Investigation. 2004;114:1218–21.
Singh AK, Tripathi P, Cardell SL. Type II NKT Cells: an elusive population with immunoregulatory properties [Internet]. Front Immunol. NLM (Medline). 2018;9:1969. https://doi.org/10.3389/fimmu.2018.01969.
Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. Cell Press. 2015;42:607–12.
Anderson MS, Bluestone JA. THE NOD MOUSE: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. https://doi.org/10.1146/annurev.immunol.23.021704.115643.
Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. https://doi.org/10.1084/jem.20040139.
Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–77.
Spence A, Purtha W, Tam J, Dong S, Kim Y, Ju CH, et al. Revealing the specificity of regulatory T cells in murine autoimmune diabetes. Proc Natl Acad Sci U S A. National Academy of Sciences. 2018;115:5265–70. https://doi.org/10.1073/pnas.1715590115.
Van Der Net JB, Bushell A, Wood KJ, Harden PN. Regulatory T cells: first steps of clinical application in solid organ transplantation [Internet]. Transpl Int. Blackwell Publishing Ltd. 2016;29:3–11. https://doi.org/10.1111/tri.12608.
Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng. 2007;96:203–9.
Funda DP, Goliáš J, Hudcovic T, Kozáková H, Špíšek R, Palová-Jelínková L. Antigen loading (e.g., glutamic acid decarboxylase 65) of tolerogenic DCs (tolDCs) reduces their capacity to prevent diabetes in the non-obese diabetes (NOD)-severe combined immunodeficiency model of adoptive cotransfer of diabetes as well as in NOD mice. Front Immunol. Frontiers Media S.A. 2018;9:290. https://doi.org/10.3389/fimmu.2018.00290.
Atkinson MA, Maclaren NK, Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. American Diabetes Association Inc. 1990;39:933–7. https://doi.org/10.2337/diab.39.8.933.
Zhang ZJ, Davidsont L, Eisenbartht G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin (diabetes/tolerance/autolmmunity/lmmunotherapy/insulin). Proc Natl Acad Sci U S A. 1991;88:10252–6.
Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23). Proc Natl Acad Sci U S A. 1996;93:956–60. https://doi.org/10.1073/pnas.93.2.956.
Liu E, Abiru N, Moriyama H, Miao D, Eisenbarth GS. Induction of insulin autoantibodies and protection from diabetes with subcutaneous insulin B:9–23 peptide without adjuvant. Ann N Y Acad Sci. 2002;958:224–7. https://doi.org/10.1111/j.1749-6632.2002.tb02974.x.
Muir A, Peck A, Clare-Salzler M, Song YH, Cornelius J, Luchetta R, et al. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-γ transcription. J Clin Invest. American Society for Clinical Investigation. 1995;95:628–34. https://doi.org/10.1172/jci117707.
Alleva DG, Gaur A, Jin L, Wegmann D, Gottlieb PA, Pahuja A, et al. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9–23) peptide. Diabetes. American Diabetes Association Inc. 2002;51:2126–34. https://doi.org/10.2337/diabetes.51.7.2126.
Coppieters KT, Harrison LC, Von Herrath MG. Trials in type 1 diabetes: Antigen-specific therapies. Clin Immunol. 2013;149:345–55. https://doi.org/10.1016/j.clim.2013.02.002.
Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561–7. https://doi.org/10.1084/jem.183.4.1561.
Petersen JS, Karlsen AE, Markholst H, Worsaae A, Dyrberg T, Micheìsen B. Neonatal tolerization with glutamic acid decarboxylase but not with bovine serum albumin delays the onset of diabetes in NOD mice. Diabetes. American Diabetes Association Inc. 1994;43:1478–84. https://doi.org/10.2337/diab.43.12.1478.
Elias D, Reshef T, Birk OS, Van Der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. National Academy of Sciences. 1991;88:3088–91. https://doi.org/10.1073/pnas.88.8.3088.
Elias D, Meilin A, Ablamunits V, Birk OS, Carmi P, Konen-Waisman S, et al. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and Downregulates autoimmunity to various -cell antigens. Diabetes. American Diabetes Association. 1997;46:758–65. https://doi.org/10.2337/diab.46.5.758.
Shoda LKM, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. Elsevier. 2005;23:115–26. https://doi.org/10.1016/j.immuni.2005.08.002.
Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. Nature Publishing Group. 2000;408:483–8. https://doi.org/10.1038/35044106.
Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Erratum: Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature (2000). 2009;Nature Publishing Group. p. 660;408:483–8. https://doi.org/10.1038/nature07964.
Elias D, Cohen IR. Peptide therapy for diabetes in NOD mice. Lancet Elsevier. 1994;343:704–6.
Bowman M, Atkinson M. Heat shock protein therapy fails to prevent diabetes in NOD mice. Diabetologia. 2002;45:1350–1. https://doi.org/10.1007/s00125-002-0897-3.
Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. The American Society for Clinical Investigation. 2006;116:2022–32. https://doi.org/10.1172/JCI28423.
Jun JC, Jones MB, Oswald DM, Sim ES, Jonnalagadda AR, Kreisman LSC, et al. T cell-intrinsic TLR2 stimulation promotes IL-10 expression and suppressive activity by CD45RbHi T cells. Rieux-Laucat F, editor. PLoS One. Public Libr Sci. 2017;12:e0180688. https://doi.org/10.1371/journal.pone.0180688.
Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. The Rockefeller University Press. 2011;208:1501–10. https://doi.org/10.1084/jem.20110574.
Serr I, Fürst RW, Achenbach P, Scherm MG, Gökmen F, Haupt F, et al. Type 1 diabetes vaccine candidates promote human Foxp3+ Treg induction in humanized mice. Nat Commun. Nature Publishing Group. 2016;7:10991. https://doi.org/10.1038/ncomms10991.
Bergman ML, Lopes-Carvalho T, Martins AC, Grieco FA, Eizirik DL, Demengeot J. Tolerogenic insulin peptide therapy precipitates type 1 diabetes. J Exp Med. 2017;214:2153–6. https://doi.org/10.1084/jem.20160471 Rockefeller University Press. Found that sustained delivery of an insulin peptide mimotope is not necessarily protective.
Daniel C, Weigmann B, von Boehmer H. Reply to “Tolerogenic insulin peptide therapy precipitates type 1 diabetes”. J Exp Med. Rockefeller University Press. 2017;214:2157–9. https://doi.org/10.1084/jem.20170285.
Mowat AMI. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. European Association for Cardio-Thoracic Surgery. 2003;3:331–41. https://doi.org/10.1038/nri1057.
Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. https://doi.org/10.1146/annurev.immunol.021908.132629.
Wilson DS, Damo M, Hirosue S, Raczy MM, Brünggel K, Diaceri G, et al. Synthetically glycosylated antigens induce antigen-specific tolerance and prevent the onset of diabetes. Nat Biomed Eng Nature Publishing Group. 2019;3:817–29. https://doi.org/10.1038/s41551-019-0424-1.
Akbarpour M, Goudy KS, Cantore A, Russo F, Sanvito F, Naldini L, et al. Insulin B chain 9–23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci Transl Med. American Association for the Advancement of Science. 2015;7:289ra81. https://doi.org/10.1126/scitranslmed.aaa3032.
Chen Y, Wu J, Wang J, Zhang W, Xu B, Xu X, et al. Targeted delivery of antigen to intestinal dendritic cells induces oral tolerance and prevents autoimmune diabetes in NOD mice. Diabetologia Springer Verlag. 2018;61:1384–96. https://doi.org/10.1007/s00125-018-4593-3.
Price JD, Tarbell KV. The role of dendritic cell subsets and innate immunity in the pathogenesis of type 1 diabetes and other autoimmune diseases. Front Immunol. Frontiers Media S.A. 2015;6:288.
Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8 + CD205 + splenic dendritic cells are specialized to induce Foxp3 + regulatory T cells. J Immunol The American Association of Immunologists. 2008;181:6923–33. https://doi.org/10.4049/jimmunol.181.10.6923.
Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T-cell tolerance and inhibit diabetes. Diabetes. 2015;64:3521–31.
Hotta-Iwamura C, Benck C, Coley WD, Liu Y, Zhao Y, Quiel JA, et al. Low CD25 on autoreactive Tregs impairs tolerance via low dose IL-2 and antigen delivery. J Autoimmun. Academic Press. 2018;90:39–48. https://doi.org/10.1016/j.jaut.2018.01.005.
Getts DR, Martin AJ, Mccarthy DP, Terry RL, Hunter ZN, Yap WT, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24.
Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, Vreden C, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. American Association for the Advancement of Science. 2014;6:219ra7. https://doi.org/10.1126/scitranslmed.3007563.
Prasad S, Neef T, Xu D, Podojil JR, Getts DR, Shea LD, et al. Tolerogenic ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J Autoimmun. Academic Press. 2018;89:112–24. https://doi.org/10.1016/j.jaut.2017.12.010.
Jamison BL, Neef T, Goodspeed A, Bradley B, Baker RL, Miller SD, et al. Nanoparticles containing an insulin–ChgA hybrid peptide protect from transfer of autoimmune diabetes by shifting the balance between effector T cells and regulatory T cells. J Immunol. 2019;203:48–57. https://doi.org/10.4049/jimmunol.1900127 The American Association of Immunologists. First approach to use neo-antigen therapeutically in T1D.
Saito E, Kuo R, Pearson RM, Gohel N, Cheung B, King NJC, et al. Designing drug-free biodegradable nanoparticles to modulate inflammatory monocytes and neutrophils for ameliorating inflammation. J Control Release. Elsevier B.V. 2019;300:185–96.
Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. Nature Publishing Group. 2016;530:434–40.
Lewis JS, Dolgova NV, Zhang Y, Xia CQ, Wasserfall CH, Atkinson MA, et al. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clin Immunol. Academic Press Inc. 2015;160:90–102. https://doi.org/10.1016/j.clim.2015.03.023.
Lewis JS, Stewart JM, Marshall GP, Carstens MR, Zhang Y, Dolgova NV, et al. Dual-sized microparticle system for generating suppressive dendritic cells prevents and reverses type 1 diabetes in the nonobese diabetic mouse model. ACS Biomater Sci Eng. 2019;5:2631–46. https://doi.org/10.1021/acsbiomaterials.9b00332Recent approach with some success reversing recent-onset T1D.
Sugarman J, Tsai S, Santamaria P, Khadra A. Quantifying the importance of pMHC valency, total pMHC dose and frequency on nanoparticle therapeutic efficacy. Immunol Cell Biol. John Wiley & Sons Ltd. 2013;91:350–9. https://doi.org/10.1038/icb.2013.9.
Han B, Serra P, Amrani A, Yamanouchi J, Marée AFM, Edelstein-Keshet L, et al. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med Nature Publishing Group. 2005;11:645–52. https://doi.org/10.1038/nm1250.
Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity Cell Press. 2010;32:568–80.
Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. The American Association of Immunologists. 2001;166:6847–54. https://doi.org/10.4049/jimmunol.166.11.6847.
A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–58.
Xia CQ, Peng R, Qiu Y, Annamalai M, Gordon D, Clare-Salzler MJ. Transfusion of apoptotic β-cells induces immune tolerance to β-cell antigens and prevents type 1 diabetes in NOD mice. Diabetes. 2007;56:2116–23. https://doi.org/10.2337/db06-0825.
Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen-coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud. Society for Biomedical Diabetes Research. 2012;9:319–27. https://doi.org/10.1900/RDS.2012.9.319.
Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1 + and IL-10–producing splenic macrophages and maintained by T regulatory cells. J Immunol. The American Association of Immunologists. 2011;187:2405–17. https://doi.org/10.4049/jimmunol.1004175.
Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9-23 epitope and involves functional epitope spreading. J Autoimmun NIH Public Access. 2012;39:347–53. https://doi.org/10.1016/j.jaut.2012.04.005.
Pishesha N, Bilate AM, Wibowo MC, Huang NJ, Li Z, Dhesycka R, et al. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc Natl Acad Sci U S A. National Academy of Sciences. 2017;114:3157–62. https://doi.org/10.1073/pnas.1701746114.
Zhou H, Sun L, Zhang S, Zhao X, Gang X, Wang G. Evaluating the causal role of gut microbiota in type 1 diabetes and its possible pathogenic mechanisms. Front Endocrinol (Lausanne). Frontiers Media S.A. 2020;11:125.
Mariño E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. Nature Publishing Group. 2017;18:552–62.
Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. Nature Publishing Group. 2018;562:589–94.
Mollah ZUA, Pai S, Moore C, O’Sullivan BJ, Harrison MJ, Peng J, et al. Abnormal NF-B function characterizes human type 1 diabetes dendritic cells and monocytes 1. J Immunol. 2008;180(5):3166–75.
Chen YG, Cabrera SM, Jia S, Kaldunski ML, Kramer J, Cheong S, et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes. American Diabetes Association Inc. 2014;63:3960–73. https://doi.org/10.2337/db14-0214.
Balmert SC, Donahue C, Vu JR, Erdos G, Falo LD, Little SR. In vivo induction of regulatory T cells promotes allergen tolerance and suppresses allergic contact dermatitis. J Control Release. Elsevier. 2017;261:223–33.
Ratay ML, Balmert SC, Acharya AP, Greene AC, Meyyappan T, Little SR. TRI microspheres prevent key signs of dry eye disease in a murine, inflammatory model. Sci Rep. Springer US. 2017;7:1–9. https://doi.org/10.1038/s41598-017-17869-y.
Fisher JD, Balmert SC, Zhang W, Schweizer R, Schnider JT, Komatsu C, et al. Treg-inducing microparticles promote donor-specific tolerance in experimental vascularized composite allotransplantation. Proc Natl Acad Sci U S A. 2019;116:25784–9.
Spanier JA, Sahli NL, Wilson JC, Martinov T, Dileepan T, Burrack AL, et al. Increased effector memory insulin-specific CD4+ T cells correlate with insulin autoantibodies in patients with recent-onset type 1 diabetes. Diabetes. American Diabetes Association Inc. 2017;66:3051–60. https://doi.org/10.2337/db17-0666.
Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia. Springer Verlag. 2017;60:1839–50. https://doi.org/10.1007/s00125-017-4377-1.
Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–7.
Phillips B, Nylander K, Harnaha J, Machen J, Lakomy R, Styche A, et al. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes. American Diabetes Association. 2008;57:1544–55. https://doi.org/10.2337/db07-0507.
Engman C, Wen Y, Meng WS, Bottino R, Trucco M, Giannoukakis N. Generation of antigen-specific Foxp3+ regulatory T-cells in vivo following administration of diabetes-reversing tolerogenic microspheres does not require provision of antigen in the formulation. Clin Immunol. Academic Press Inc. 2015;160:103–23. https://doi.org/10.1016/j.clim.2015.03.004.
Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. National Academy of Sciences. 2010;107:20768–73. https://doi.org/10.1073/pnas.1009201107.
Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109:11270–5. https://doi.org/10.1073/pnas.1120611109.
Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal. American Association for the Advancement of Science. 2016;9:ra61. https://doi.org/10.1126/scisignal.aad0612.
Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Matter. Nature Publishing Group. 2009;8:151–8. https://doi.org/10.1038/nmat2357.
Verbeke CS, Gordo S, Schubert DA, Lewin SA, Desai RM, Dobbins J, Wucherpfennig KW, Mooney DJ Multicomponent injectable hydrogels for antigen-specific Tolerogenic immune modulation. Adv Healthc Mater. Wiley-VCH Verlag; 2017;6. https://doi.org/10.1002/adhm.201600773.
Husseiny MI, Du W, Mbongue J, Lenz A, Rawson J, Kandeel F, et al. Factors affecting Salmonella-based combination immunotherapy for prevention of type 1 diabetes in non-obese diabetic mice. Vaccine. Elsevier Ltd. 2018;36:8008–18.
Mbongue JC, Rawson J, Garcia PA, Gonzalez N, Cobb J, Kandeel F, et al. Reversal of new onset type 1 diabetes by oral salmonella-based combination therapy and mediated by regulatory T-cells in NOD mice. Front Immunol. 2019;10:320. https://doi.org/10.3389/fimmu.2019.00320 Frontiers Media S.A. Recent approach with some success reversing recent-onset T1D.
Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. Presse Dienstleistungsgesellschaft mbH und Co. KG. 2000;6:1176–82. https://doi.org/10.1038/80525.
Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. Nature Publishing Group. 2000;6:1167–75. https://doi.org/10.1038/80516.
Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crinò A, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). Diabetologia. 2000;43:1000–4. https://doi.org/10.1007/s001250051482.
Skyler JS. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial-type 1. Diabetes Care. American Diabetes Association. 2005;28:1068–76. https://doi.org/10.2337/diacare.28.5.1068.
Krischer J, Greenbaum C, Atkinson M, Baidal D, Battaglia M, Becker D, et al. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: A randomized clinical trial. JAMA - J Am Med Assoc. American Medical Association. 2017;318:1891–902. https://doi.org/10.1001/jama.2017.17070.
Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. American Diabetes Association. 2011;60:1237–45. https://doi.org/10.2337/db10-1360.
Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R. No effect of the altered peptide ligand NBI-6024 on β-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care. 2009;32:2036–40. https://doi.org/10.2337/dc09-0449.
Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. Massachussetts Medical Society. 2012;366:433–42. https://doi.org/10.1056/NEJMoa1107096.
Wherrett DK, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: A randomised double-blind trial. Lancet. Lancet Publishing Group. 2011;378:319–27. https://doi.org/10.1016/S0140-6736(11)60895-7.
Lazar L, Ofan R, Weintrob N, Avron A, Tamir M, Elias D, et al. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev. 2007;23:286–91. https://doi.org/10.1002/dmrr.711.
Schloot NC, Meierhoff G, Lengyel C, Vándorfi G, Takács J, Pánczél P, et al. Effect of heat shock protein peptide DiaPep277 on β-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev. 2007;23:276–85. https://doi.org/10.1002/dmrr.707.
Carballido JM, Santamaria P. Taming autoimmunity: translating antigen-specific approaches to induce immune tolerance. J Exp Med. Rockefeller University Press. 2019;216:247–50. https://doi.org/10.1084/jem.20182287.
Acknowledgments
The figure was created with BioRender.
Funding
E.J.B. was supported in part by a fellowship (T32-AI074490) from the NIH National Institute of Allergy and Infectious Diseases and the ARCS Foundation Scholar Award. The funders had no role in the decision to publish or the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.R.L. and J.D.P. are both corresponding authors for the manuscript. E.J.B. conceived of the review, and all authors contributed to writing and editing the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Steven R. Little is an inventor on patent 14/372,977 entitled “Controlled Release Formulations for the Induction and Proliferation of Blood Cells” which was issued to the University of Pittsburgh on 04/30/20. This patent covers the TRI MP formulation discussed in this manuscript.
Ethan J. Bassin and Jon D. Piganelli each declare they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pathogenesis of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Bassin, E.J., Piganelli, J.D. & Little, S.R. Auto-antigen and Immunomodulatory Agent–Based Approaches for Antigen-Specific Tolerance in NOD Mice. Curr Diab Rep 21, 9 (2021). https://doi.org/10.1007/s11892-021-01376-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11892-021-01376-6