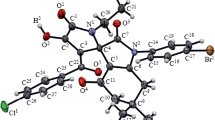
The three-component reaction of (E)-1,5-diarylpent-4-ene-1,3-diones, isatins, and (thia)proline in i-PrOH at room temperature proceeds regio- and stereoselectively leading to the formation of spiro[(thia)pyrrolizidine-3,3'-oxindoles] with the 1,3-dicarbonyl moiety in the (thia)pyrrolizidine ring. Subsequent treatment of these tetracyclic products with arylhydrazine hydrochlorides under reflux in the EtOH–AcOH system gave 5-substituted 1,3-diaryl-1H-pyrazoles in high yields.



Similar content being viewed by others
References
(a) Arrieta, A.; Beyer, L.; Kleinpeter, E.; Lehmann, J.; Dargatz, M. J. Prakt. Chem. 1992, 334, 696. (b) Reddy, B. P.; Krupadanam, G. L. D. J. Heterocycl. Chem. 1996, 33, 1561. (c) Linn, B. O.; Hauser, C. R. J. Am. Chem. Soc. 1956, 78, 6066.
(a) Jang, Y.-J.; Chen, Y.-S.; Lee, C.-J.; Chen, C.-H.; Lin, W. Synthesis 2015, 95. (b) Chen, C.-H.; Ko, C.-T.; Reddy, G. M.; Lee, C.-J.; Lin, W. Eur. J. Org. Chem. 2015, 5254.
(a) MacDonald, F. K.; Burnell, D. J. J. Org. Chem. 2009, 74, 6973. (b) Saulnier, S.; Lozovskiy, S. V.; Golovanov, A. A.; Ivanov, A. Yu.; Vasilyev, A. V. Eur. J. Org. Chem. 2017, 3635.
(a) Makrandi, J. K.; Kumari, V. Synth. Commun. 1989, 19, 1919. (b) Pinto, J.; Silva, V. L. M.; Silva, A. M. G.; Silva, A. M. S. Molecules 2015, 20, 11418.
Letcher, R. M.; Yue, T.-Y.; Chiu, K.-F.; Kelkar, A. S.; Cheung, K.-K. J. Chem. Soc., Perkin Trans. 1 1998, 3267.
(a) Selvam, C.; Jachak, S. M.; Thilagavathi, R.; Chakraborti, A. K. Bioorg. Med. Chem. Lett. 2005, 15, 1793. (b) Caldarelli, A.; Penucchini, E.; Caprioglio, D.; Genazzani, A. A.; Minassi, A. Bioorg. Med. Chem. 2013, 21, 5510. (c) Nieto, C. I.; Cabildo, M. P.; Cornago, M. P.; Sanz, D.; Claramunt, R. M.; Torralba, M. C.; Torres, M. R.; Elguero, J.; García, J. A.; López, A.; Acuña-Castroviejo, D. Molecules 2015, 20, 15643.
(a) Liou, Y.-C.; Su, Y.-H.; Ku, K.-C.; Edukondalu, A.; Lin, C.-K.; Ke, Y.-S.; Karanam, P.; Lee, C.-J.; Lin, W. Org. Lett. 2019, 21, 8339. (b) Sousa, J. L. C.; Talhi, O.; Rocha, D. H. A.; Pinto, D. C. G. A.; Paz, F. A. A.; Bachari, K.; Kirsch, G.; Silva, A. M. S. Synlett 2015, 2724.
(a) Nájera, C.; Sansano, J. M. Pure Appl. Chem. 2019, 91, 575. (b) Mei, G.-J.; Shi, F. Chem. Commun. 2018, 54, 6607. (c) Döndas, H. A.; de Gracia Retamosa, M.; Sansano, J. M. Synthesis 2017, 2819. (d) Pavlovska, T. L.; Redkin, R. Gr.; Lipson, V. V.; Atamanuk, D. V. Mol. Diversity 2016, 20, 299. (e) Yan, L.-J.; Wang, Y.-C. ChemistrySelect 2016, 1, 6948. (f) Lashgari, N.; Ziarani, G. M. ARKIVOC 2012, (i), 277. (g) Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. Expert Opin. Drug Discovery 2020, 15, 603. (h) Ye, N.; Chen, H.; Wold, E. A.; Shi, P. Y.; Zhou, J. ACS Infect. Dis. 2016, 2, 382. (i) Yu, B.; Yu, D.-Q.; Liu, H.-M. Eur. J. Med. Chem. 2015, 97, 673. (j) Santos, M. M. M. Tetrahedron 2014, 70, 9735. (k) Beloglazkina, A. A.; Karpov, N. A.; Mefedova, S. R.; Polyakov, V. S.; Skvortsov, D. A.; Kalinina, M. A.; Tafeenko, V. A.; Majouga, A. G.; Zyk, N. V.; Beloglazkina, E. K. Russ. Chem. Bull., Int. Ed. 2019, 68, 1006. [Izv. Akad. Nauk, Ser. Khim. 2019, 1006.]
(a) Jossang, A.; Jossang, P.; Hadi, H. A.; Sévenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527. (b) Kornet, M. J.; Thio, A. P. J. Med. Chem. 1976, 19, 892. (c) Kang, T.-H.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H.; Matsumoto, K. Life Sci. 2004, 76, 331. (d) Kathirvelan, D.; Haribabu, J.; Reddy, B. S. R.; Balachandran, C.; Duraipandiyan, V. Bioorg. Med. Chem. Lett. 2015, 25, 389. (e) Barakat, A.; Islam, M. S.; Ghawas, H. M.; Ghawas, H. M.; Al-Majid, A. M.; El-Senduny, F. F.; Badria, F. A.; Elshaier, Y. A. M. M.; Ghabbour, H. A. Bioorg. Chem. 2019, 86, 598.
Zimnitskiy, N. S.; Denikaev, A. D.; Barkov, A. Y.; Kutyashev, I. B.; Korotaev, V. Y.; Sosnovskikh, V. Y. J. Org. Chem. 2020, 85, 8683.
(a) Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Kutyashev, I. B.; Moshkin, V. S.; Sosnovskikh, V. Ya. Tetrahedron 2016, 72, 6825. (b) Kutyashev, I. B.; Barkov, A. Yu.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2019, 55, 529. [Khim. Geterotsikl. Soedin. 2019, 55, 529.] (c) Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. New J. Chem. 2019, 43, 18495. c Kutyashev, I. B.; Kochnev, I. A.; Cherepkova, A. A.; Zimnitskiy, N. S.; Barkov, A. Yu.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2020, 56, 1302. [Khim. Geterotsikl. Soedin. 2020, 56, 1302.]
Izmest'ev, A. N.; Gazieva, G. А.; Kravchenko, A. N. Chem. Heterocycl. Compd. 2020, 56, 255. [Khim. Geterotsikl. Soedin. 2020, 56, 255.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2015, A71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(1), 81–91
Rights and permissions
About this article
Cite this article
Korotaev, V.Y., Zimnitskiy, N.S., Denikaev, A.D. et al. 1,5-Diarylpent-4-ene-1,3-diones in the synthesis of spiro[(thia)pyrrolizidine-3,3'-oxindoles] and 1,3-diaryl-5-spiro[oxindole-3,3'-pyrrolizidin-2'-yl]-1H-pyrazoles. Chem Heterocycl Comp 57, 81–91 (2021). https://doi.org/10.1007/s10593-021-02871-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02871-0