Abstract
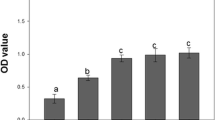
The tritrophic interactions among plant virus, host plants, and insect vectors directly influence the natural ecosystem, which, in turn, has tremendous practical implications in the sustainable pest control strategies. Cucumber mosaic virus (CMV), transmitted by aphids in a non-persistent manner, causes severe damage in diverse crops worldwide. There is a wealth of information on the initial round of interactions within this tritrophic system. However, knowledge on the subsequent round of interactions is very limited. In this research, we focused on their interactions among Nicotiana tabacum cv. Samsum plants, CMV, and green peach aphids, Myzus persicae specifically after the initial round of aphid feeding on CMV-infected plants. Our results show that initial aphid feeding on CMV-infected plants reduces the fitness of the subsequent aphids. The reproduction capacity, longevity, and survival rate of M. persicae are reduced on CMV-infected plants, previously foraged by M. persicae. Furthermore, the initial aphid feeding on CMV-infected plants induce gene expression involved in the salicylic acid (SA) signaling pathway and suppresses the expression of downstream genes associated with jasmonic acid (JA) signaling pathway. Besides, plant chlorophyll content and nitrogen source are reduced on those CMV-infected plants, previously foraged by aphids. The negative impacts on the fitness and performance of the subsequent aphids may have significant implications in virus transmission, distribution, and epidemiology.






Similar content being viewed by others
References
Avila CA et al (2012) Loss of function of FATTY ACID DESATURASE7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol 158:2028
Bak A, Patton M, Perilla-Henao L, Aegerter B, Casteel C (2019) Ethylene signaling mediates potyvirus spread by aphid vectors. Oecologia 190:139–148
Bera S, Blundell R, Liang D, Crowder DW, Casteel CL (2020) The oxylipin signaling pathway is required for increased aphid attraction and retention on virus-infected plants. J Chem Ecol 46:771–781
Blanc S, Michalakis Y (2016) Manipulation of hosts and vectors by plant viruses and impact of the environment. Curr Opin Insect Sci 16:36–43
Carmo-Sousa M, Moreno A, Garzo E, Fereres A (2014) A non-persistently transmitted-virus induces a pull-push strategy in its aphid vector to optimize transmission and spread. Virus Res 186:38–46
Carr J, Murphy A, Tungadi T, Yoon J (2019) Plant defense signals: players and pawns in plant-virus–vector interactions. Plant Sci 279:87–95
Casteel CL, De Alwis M, Bak A, Dong H, Whitham SA, Jander G (2015) Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the aphid vector, Myzus persicae. Plant Physiol 169:209–218
Chrétien L et al (2018) Caterpillars induce jasmonates in flowers and alter plant responses to a second attacker. New Phytol 217:1279–1291
Dethier VG (2010) Food-aversion learning in two polyphagous caterpillars, Diacrisia virginica and Estigmene congrua. Physiol Entomol 5:321–325
Dietzgen RG, Mann KS, Johnson KN (2016) Plant virus–insect vector interactions: current and potential future research directions. Viruses 8:303
Donnelly R, Cunniffe NJ, Carr JP, Gilligan CA (2019) Pathogenic modification of plants enhances long-distance dispersal of nonpersistently transmitted viruses to new hosts. Ecology 100:e02725
Fu X, Ye L, Kang L, Ge F (2010) Elevated CO2 shifts the focus of tobacco plant defences from cucumber mosaic virus to the green peach aphid. Plant Cell Environ 33:2056–2064
Gilbertson R, Batuman O, Webster C, Adkins S (2015) Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu Rev Virol 2:67–93
Guo H, Gu L, Liu F, Chen F, Ge F, Sun Y (2019) Aphid-borne viral spread is enhanced by virus-induced accumulation of plant reactive oxygen species. Plant Physiol 179:143–155
He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128:876–884
Ingwell LL, Eigenbrode SD, Bosque-Perez NA (2012) Plant viruses alter insect behavior to enhance their spread. Sci Rep 2:578
Kloth K et al (2016) AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J Exp Bot 67:3383–3396
Lewsey MG et al (2010) Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol Plant Microbe Interact 23:835–845
Li Y, Nan Z, Duan T (2019) Rhizophagus intraradices promotes alfalfa (Medicago sativa) defense against pea aphids (Acyrthosiphon pisum) revealed by RNA-Seq analysis. Mycorrhiza 29:623–635
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Mauck K, Chesnais Q (2020) A synthesis of virus–vector associations reveals important deficiencies in studies on host and vector manipulation by plant viruses. Virus Res 285:197957
Mauck KE, De Moraes CM, Mescher MC (2010) Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci USA 107:3600–3605
Mauck K, Bosque-Pérez NA, Eigenbrode SD, Moraes CMD, Mescher MC (2012) Transmission mechanisms shape pathogen effects on host–vector interactions: evidence from plant viruses. Funct Ecol 26:1162–1175
Mauck K, De Moraes C, Mescher M (2014) Evidence of local adaptation in plant virus effects on host–vector interactions. Integr Comp Biol 54:193–209
Mauck KE, Moraes CMD, Mescher MC (2015) Infection of host plants by cucumber mosaic virus increases the susceptibility of Myzus persicae aphids to the parasitoid Aphidius colemani. Sci Rep 5:10963
Moeini P, Afsharifar A, Izadpanah K, Sadeghi S, Eigenbrode S (2020) Maize iranian mosaic virus (family Rhabdoviridae) improves biological traits of its vector Laodelphax striatellus. Arch Virol 165:169–178
Patton M, Bak A, Sayre J, Heck M, Casteel C (2020) A polerovirus, potato leafroll virus, alters plant–vector interactions using three viral proteins. Plant Cell Environ 43:387–399
Sarmento RA et al (2011) A herbivore that manipulates plant defence. Ecol Lett 14:229–236
Shapiro L, Moraes CMD, Stephenson AG, Mescher MC (2012) Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol Lett 15:1430–1438
Shi X et al (2014a) Three-way interactions between the tomato plant, tomato yellow leaf curl virus, and Bemisia tabaci (Hemiptera: Aleyrodidae) facilitate virus spread. J Econ Entomol 107:920–926
Shi X et al (2014b) Bemisia tabaci Q carrying tomato yellow leaf curl virus strongly suppresses host plant defenses. Sci Rep 4:5230
Shi X, Gao Y, Yan S, Tang X, Zhou X, Zhang D, Liu Y (2016) Aphid performance changes with plant defense mediated by cucumber mosaic virus titer. Virol J 13:70
Shrestha A, Sundaraj S, Culbreath A, Riley D, Abney M, Srinivasan R (2015) Effects of thrips density, mode of inoculation, and plant age on tomato spotted wilt virus transmission in peanut plants. Environ Entomol 44:136–143
Su Q et al (2015) Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J Econ Entomol 108:11–19
Sugio A, Kingdom HN, Maclean AM (2011) Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci USA 108:1254–1263
Tack AJM, Dicke M (2013) Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct Ecol 27:633–645
Tu Z, Ling B, Xu D, Zhang M, Zhou G (2013) Effects of southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol J 10:145
Tungadi T et al (2017) Cucumber mosaic virus and its 2b protein alter emission of host volatile organic compounds but not aphid vector settling in tobacco. Virol J 14:91
Vinoth Kumar R, Shivaprasad P (2020) Plant-virus–insect tritrophic interactions: insights into the functions of geminivirus virion-sense strand genes. Proc Biol Sci 287:1936
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306
Westwood JH et al (2013) A trio of viral proteins tunes aphid-plant interactions in Arabidopsis thaliana. PLoS ONE 8:e83066
Wu D et al (2017) Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res 27:402–415
Yates-Stewart A, Pekarcik A, Michel A, Blakeslee J (2020) Jasmonic acid-isoleucine (JA-Ile) is involved in the host-plant resistance mechanism against the soybean aphid (Hemiptera: Aphididae). J Econ Entomol 9:1–7
Zhang H, Bernonville TDD, Body M, Glevarec G, Giron D (2015) Leaf-mining by Phyllonorycter blancardella reprograms the host-leaf transcriptome to modulate phytohormones associated with nutrient mobilization and plant defense. J Insect Physiol 84:114–127
Zhang N, Zhou S, Yang D, Fan Z (2020) Revealing shared and distinct genes responding to JA and SA signaling in Arabidopsis by meta-analysis. Front Plant Sci 11:908
Zhao J, Liu X, Fang Y, Fang R, Guo H (2018) CMV2b-dependent regulation of host defense pathways in the context of viral infection. Viruses 10:618
Zheng X et al (2019) Tripartite interactions between jasmonic/salicylic acid pathways, western flower thrips, and thrips-transmitted tomato zonate spot virus infection in Capsicuum annuum. Arthropod-Plant Interact 13:289–297
Zhu F, Xi DH, Yuan S, Xu F, Zhang DW, Lin HH (2014) Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol Plant Microbe Interact 27:567
Ziebell H et al (2011) Cucumber mosaic virus and its 2b RNA silencing suppressor modify plant-aphid interactions in tobacco. Sci Rep 1:187
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31872932, 31571981), the National Key R&D Program of China (2018YFE0112600), the Agriculture Research System of China (CARS-23-D-02), the National Agricultural Outstanding Talent Program ([2015]62), and the Hunan Natural Science Foundation (2019JJ30014).
Author information
Authors and Affiliations
Contributions
XBS, DYZ, and YL designed the experiment. XBS and SY carried the experimental work. JD, ZZ, LMZ, SES, YG, and XGZ contributed reagents/materials. XBS wrote the paper.
Corresponding authors
Additional information
Handling Editor: Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, Xb., Deng, J., Zhang, Z. et al. Initial ingestion of CMV-infected plants reduces subsequent aphid performance. Arthropod-Plant Interactions 15, 153–160 (2021). https://doi.org/10.1007/s11829-021-09804-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09804-w