Abstract
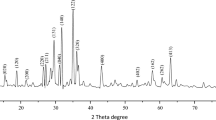
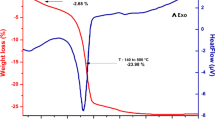
In this study, CuO–ZrO2 nanoparticles (ZrO2 modified by copper(II)oxide) were prepared by using the impregnation method. The physicochemical properties of produced nanoparticles were characterized using differential reflectance spectra, X-ray diffraction, field-emission scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy. The occurrence of copper(II) was confirmed by XPS technique. The calculated average crystallite size was determined by TEM spectroscopy. The DRS studies exhibited a reduction in the band gap of ZrO2 from 4.7 to 2.9 eV after modification with copper(II) oxide. The photocatalytic method with sunlight was used to examine the ability of copper(II) oxide-modified zirconium(IV) oxide nanoparticles in removing toxic chemicals such as rhodamine B (RhB) and methylene blue (MB). Based on the analysis of experiments, copper(II)oxide-modified ZrO2 showed a good photocatalytic activity, such that 1 mg of the photocatalyst degraded 10 ppm of MB and RhB within 30 and 60 min, respectively. The degradation of MB and RhB dyes was tested four and three times, respectively, to ensure reusability of photocatalyst.
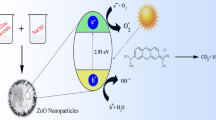
Graphic abstract
Copper(II)oxide-modified zirconium(IV) oxide nanoparticles were examined in regard to their ability in removing toxic chemicals such as rhodamine B (RhB) and methylene blue (MB) through the use of the photocatalytic method. Based on the analysis of experiments, copper(II)oxide-modified ZrO2 showed a good photocatalytic activity, such that 1 mg of the photocatalyst degraded 10 ppm dye within 30 and 60 min for MB and RhB, respectively.












Similar content being viewed by others
References
C.V. Reddy, I.N. Reddy, B. Akkinepally, V.V. Harish, K.R. Reddy, S. Jaesool, Ceram. Int. 45(12), 15298–306 (2019)
K. Murugesan, A. Dhamija, I.H. Nam, Y.M. Kim, Y.S. Chang, Dyes Pigments. 75, 176 (2007)
N. Willmott, J. Guthrie, G. Nelson, J. Soc. Dyers Colour 114, 38 (1998)
S. Jian, S. Sun, Y. Zeng, Z. Liu, Y. Liu, Q. Yang, G. Ma, Appl. Surf. Sci. 505, 144318 (2020)
H. Liu, W. Guo, Y. Li, S. He, C. He, J. Environ. Chem. Eng. 6(1), 59–67 (2018)
D. P. Loucks, E. van Beek. Water Resource Systems Planning and Management (Springer, 2017), pp. 1–49
K. Siwińska-Ciesielczyk, D. Świgoń, P. Rychtowski, D. Moszyński, A. Zgoła-Grześkowiak, T. Jesionowski, Coll. Surf, A: Physicochem. Eng. Asp. 586, 124272 (2020)
A.H. Kianfar, M.A. Arayesh, J. Environ. Chem. Eng. 26, 103640 (2019)
G. Crini, E. Lichtfouse, Environ. Chem. Lett. 17(1), 145–55 (2019)
N. Doufar, M. Benamira, H. Lahmar, M. Trari, I. Avramova, M.T. Caldes, J. Photochem. Photobiol. A: Chem. 26, 112105 (2019)
K. Ghorai, A. Panda, M. Bhattacharjee, D. Mandal, A. Hossain, P. Bera, M.M. Seikh, A. Gayen, Appl. Surf. Sci. 8, 147604 (2020)
H. Li, Z. Wang, Y. Lu, S. Liu, X. Chen, G. Wei, G. Ye, J. Chen, Appl. Surf. Sci. 531, 147307 (2020)
P.K. Robertson, J. Clean. Product. 4, 203–12 (1996)
S. Das, V.C. Srivastava, Photochem. Photobiol. Sci. 15(6), 714–730 (2016)
L. Dashairya, M. Sharma, S. Basu, P. Saha, J. Alloy. Compd. 25(735), 234–245 (2018)
S. Polisetti, P.A. Deshpande, G. Madras, Ind. Eng. Chem. Res 50(23), 12915–12924 (2011)
N.J. Rahman, A. Ramli, K. Jumbri, Y. Uemura, Sci. Reports. 9(1), 1–2 (2019)
S. Li, J. He, Y. Dan, X. Li, Y. Jiao, J. Deng, J. Wang, Y. Chen, L. Jiang, Mater. Chem.Phys. 15(240), 122150 (2020)
V.C. Ho, S. Jeong, T. Yim, J. Mun, J. Power Sour. 29(450), 227625 (2020)
K.L. He Zheng, H. Cao, X. Zhang, J. Phys. Chem. C 113, 18259–18263 (2009)
V. Melchor-Lagar, E. Ramos-Ramírez, A.A. Morales-Pérez, I. Rangel-Vázquez, G. Del Angel, J. Photochem. Photobiol. A: Chem. 5(389), 112251 (2020)
L. Renuka, K.S. Anantharaju, Y.S. Vidya, H.P. Nagaswarupa, S.C. Prashantha, S.C. Sharma, H. Nagabhushana, G.P. Darshan, Appl. Catal. B 5(210), 97–115 (2017)
R. Gopal, A. Sambandam, T. Kuppulingam, S. Meenakshisundaram, M.S. AlSalhi, S. Devanesan, J. Mater. Sci.: Mater. Electron. 1, 1–5 (2020)
R. Zhang, Y. Ma, W. Lan, D.E. Sameen, S. Ahmed, J. Dai, W. Qin, S. Li, Y. Liu, Ultrason. Sonochem. 10, 105343 (2020)
Y. Bessekhouad, D. Robert, J.V. Weber, N. Chaoui, J. Photochem. Photobiol. A 167(1), 49–57 (2004)
X. Zhang, S. Wei, X. Zhao, Z. Chen, H. Wu, P. Rong, Y. Sun, Y. Li, H. Yu, D. Wang, Appl. Catal. A: General. 25(590), 117313 (2020)
Z. He, M. Li, Y. Li, C. Li, Z. Yi, J. Zhu, L. Dai, W. Meng, H. Zhou, L. Wang, Electrochim. Acta 20(309), 166–176 (2019)
B. Li, Zhang W. J. Alloy. Compd. 26, 153158 (2019)
C. Liu, X. Li, L. Zhang, X. Yuan, Y. Wu, X. Wang. J. Mater. Sci.: Mater. Electron 31(17), 14221–14232 (2020)
C.V. Reddy, I.N. Reddy, K. Ravindranadh, K.R. Reddy, D. Kim, J. Shim, Sep. Purif. Technol. 17, 117352 (2020)
J. Abdi, M. Yahyanezhad, S. Sakhaie, M. Vossoughi, I. Alemzadeh, J. Environ. Chem. Eng. 7(3), 103096 (2019)
F. Vahedi Gerdeh, A. Feizbakhsh, E. Konoz, H. Faraji, Int. J. Environ. Anal. Chem. 16, 1–5 (2020)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kianfar, A.H., Arayesh, M.A. & Momeni, M.M. Degradation of MB and RhB by modified ZrO2 nanoparticles via sunlight. Appl. Phys. A 127, 158 (2021). https://doi.org/10.1007/s00339-020-04257-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04257-z