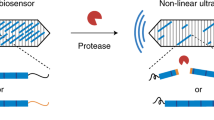
Abstract
Many questions in basic biology and medicine require the ability to visualize the function of specific cells and molecules inside living organisms. In this context, technologies such as ultrasound, optoacoustics and magnetic resonance provide non-invasive imaging access to deep-tissue regions, as used in many laboratories and clinics to visualize anatomy and physiology. In addition, recent work has enabled these technologies to image the location and function of specific cells and molecules inside the body by coupling the physics of sound waves, nuclear spins and light absorption to unique protein-based materials. These materials, which include air-filled gas vesicles, capsid-like nanocompartments, pigment-producing enzymes and transmembrane transporters, enable new forms of biomolecular and cellular contrast. The ability of these protein-based contrast agents to be genetically encoded and produced by cells creates opportunities for unprecedented in vivo studies of cellular function, while their amenability to genetic engineering enables atomic-level design of their physical, chemical and biological properties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

panel e adapted with permission from ref. 22, Springer Nature Ltd.
Similar content being viewed by others
References
Piraner, D. I. et al. Going deeper: biomolecular tools for acoustic and magnetic imaging and control of cellular function. Biochemistry 56, 5202–5209 (2017).
Marblestone, A. H. et al. Physical principles for scalable neural recording. Front. Comput. Neurosci. 7, 137 (2013).
Wang, L. V. & Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 13, 627–638 (2016).
Maresca, D. et al. Biomolecular ultrasound and sonogenetics. Annu. Rev. Chem. Biomol. Eng. 9, 229–252 (2018).
Mukherjee, A., Davis, H. C., Ramesh, P., Lu, G. J. & Shapiro, M. G. Biomolecular MRI reporters: evolution of new mechanisms. Prog. Nucl. Magn. Reson. Spectrosc. 102–103, 32–42 (2017).
Paefgen, V., Doleschel, D. & Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 6, 197 (2015).
Wahsner, J., Gale, E. M., Rodríguez-Rodríguez, A. & Caravan, P. Chemistry of MRI contrast agents: current challenges and new frontiers. Chem. Rev. 119, 957–1057 (2019).
Chung, J.-K. Sodium iodide symporter: its role in nuclear medicine. J. Nucl. Med. 43, 1188–1200 (2002).
Kircher, M. F., Gambhir, S. S. & Grimm, J. Noninvasive cell-tracking methods. Nat. Rev. Clin. Oncol. 8, 677–688 (2011).
Louie, A. Y. et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat. Biotechnol. 18, 321–325 (2000).
Genove, G., DeMarco, U., Xu, H., Goins, W. F. & Ahrens, E. T. A new transgene reporter for in vivo magnetic resonance imaging. Nat. Med. 11, 450–454 (2005).
Cohen, B., Dafni, H., Meir, G., Harmelin, A. & Neeman, M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia 7, 109–117 (2005).
Duewell, S., Kasserra, C. E., Jezzard, P. & Balaban, R. S. Evaluation of methemoglobin as an autologous intravascular MRI contrast agent. Magn. Reson. Med. 35, 787–789 (1996).
Shapiro, M. G. et al. Directed evolution of a magnetic resonance imaging contrast agent for noninvasive imaging of dopamine. Nat. Biotechnol. 28, 264–270 (2010).
Yang, J. J. et al. Rational design of protein-based MRI contrast agents. J. Am. Chem. Soc. 130, 9260–9267 (2008).
Deans, A. E. et al. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn. Reson. Med. 56, 51–59 (2006).
Patrick, P. S. et al. Dual-modality gene reporter for in vivo imaging. Proc. Natl Acad. Sci. USA 111, 415–420 (2014).
Gilad, A. A. et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat. Biotechnol. 25, 217–219 (2007).
Yuan, Y. et al. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 18, 1376–1383 (2019).
Mukherjee, A., Wu, D., Davis, H. C. & Shapiro, M. G. Non-invasive imaging using reporter genes altering cellular water permeability. Nat. Commun. 7, 13891 (2016).
Schilling, F. et al. MRI measurements of reporter-mediated increases in transmembrane water exchange enable detection of a gene reporter. Nat. Biotechnol. 35, 75–80 (2017).
Desai, M., Slusarczyk, A. L., Chapin, A., Barch, M. & Jasanoff, A. Molecular imaging with engineered physiology. Nat. Commun. 7, 13607 (2016).
Ohlendorf, R. et al. Target-responsive vasoactive probes for ultrasensitive molecular imaging. Nat. Commun. 11, 2399 (2020).
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Shu, X. et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science 324, 804–807 (2009).
Filonov, G. S. et al. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 29, 757–761 (2011).
Fuenzalida Werner, J. P. et al. Structure-based mutagenesis of phycobiliprotein smURFP for optoacoustic imaging. ACS Chem. Biol. 14, 1896–1903 (2019).
Stiel, A. C. et al. High-contrast imaging of reversibly switchable fluorescent proteins via temporally unmixed multispectral optoacoustic tomography. Opt. Lett. 40, 367–370 (2015).
Deán-Ben, X. L. et al. Light fluence normalization in turbid tissues via temporally unmixed multispectral optoacoustic tomography. Opt. Lett. 40, 4691–4694 (2015).
Yao, J. et al. Multiscale photoacoustic tomography using reversibly switchable bacterial phytochrome as a near-infrared photochromic probe. Nat. Methods 13, 67–73 (2016).
Deán-Ben, X. L. et al. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci. Appl. 5, e16201 (2016).
Qian, Y. et al. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat. Methods 16, 171–174 (2019).
Jutz, G., van Rijn, P., Santos Miranda, B. & Böker, A. Ferritin: a versatile building block for bionanotechnology. Chem. Rev. 115, 1653–1701 (2015).
Gossuin, Y., Gillis, P., Hocq, A., Vuong, Q. L. & Roch, A. Magnetic resonance relaxation properties of superparamagnetic particles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 1, 299–310 (2009).
Iordanova, B., Robison, C. S. & Ahrens, E. T. Design and characterization of a chimeric ferritin with enhanced iron loading and transverse NMR relaxation rate. J. Biol. Inorg. Chem. 15, 957–965 (2010).
Matsumoto, Y., Chen, R., Anikeeva, P. & Jasanoff, A. Engineering intracellular biomineralization and biosensing by a magnetic protein. Nat. Commun. 6, 8721 (2015).
Liu, X. et al. Engineering genetically-encoded mineralization and magnetism via directed evolution. Sci. Rep. 6, 38019 (2016).
Douglas, T. et al. Protein engineering of a viral cage for constrained nanomaterials synthesis. Adv. Mater. 14, 415–418 (2002).
McHugh, C. A. et al. A virus capsid-like nanocompartment that stores iron and protects bacteria from oxidative stress. EMBO J. 33, 1896–1911 (2014).
He, D. et al. Structural characterization of encapsulated ferritin provides insight into iron storage in bacterial nanocompartments. eLife 5, e18972 (2016).
Giessen, T. W. & Silver, P. A. Widespread distribution of encapsulin nanocompartments reveals functional diversity. Nat. Microbiol. 2, 17029 (2017).
Sigmund, F. et al. Bacterial encapsulins as orthogonal compartments for mammalian cell engineering. Nat. Commun. 9, 1990 (2018).
Sigmund, F. et al. Iron-sequestering nanocompartments as multiplexed electron microscopy gene reporters. ACS Nano 13, 8114–8123 (2019).
Ramesh, P. et al. Ultraparamagnetic cells formed through intracellular oxidation and chelation of paramagnetic iron. Angew. Chem. Int. Ed. Engl. 57, 12385–12389 (2018).
Komeili, A. Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria. FEMS Microbiol. Rev. 36, 232–255 (2012).
Kolinko, I. et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat. Nanotechnol. 9, 193–197 (2014).
Stritzker, J. et al. Vaccinia virus-mediated melanin production allows MR and optoacoustic deep tissue imaging and laser-induced thermotherapy of cancer. Proc. Natl Acad. Sci. USA 110, 3316–3320 (2013).
Jiang, Y. et al. Violacein as a genetically-controlled, enzymatically amplified and photobleaching-resistant chromophore for optoacoustic bacterial imaging. Sci. Rep. 5, 11048 (2015).
Lauri, A. et al. Whole-cell photoacoustic sensor based on pigment relocalization. ACS Sens. 4, 603–612 (2019).
Shapiro, M. G. et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol. 9, 311–316 (2014).
Maresca, D. et al. Nonlinear ultrasound imaging of nanoscale acoustic biomolecules. Appl. Phys. Lett. 110, 073704 (2017).
Maresca, D., Sawyer, D. P., Renaud, G., Lee-Gosselin, A. & Shapiro, M. G. Nonlinear X-wave ultrasound imaging of acoustic biomolecules. Phys. Rev. X 8, 041002 (2018).
Lakshmanan, A. et al. Molecular engineering of acoustic protein nanostructures. ACS Nano 10, 7314–7322 (2016).
Cherin, E. et al. Acoustic behavior of halobacterium salinarum gas vesicles in the high-frequency range: experiments and modeling. Ultrasound Med. Biol. 43, 1016–1030 (2017).
Lakshmanan, A. et al. Preparation of biogenic gas vesicle nanostructures for use as contrast agents for ultrasound and MRI. Nat. Protoc. 12, 2050–2080 (2017).
Bourdeau, R. W. et al. Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts. Nature 553, 86–90 (2018).
Riglar, D. T. & Silver, P. A. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 16, 214–225 (2018).
Farhadi, A., Ho, G. H., Sawyer, D. P., Bourdeau, R. W. & Shapiro, M. G. Ultrasound imaging of gene expression in mammalian cells. Science 365, 1469–1475 (2019).
Lakshmanan, A. et al. Acoustic biosensors for ultrasound imaging of enzyme activity. Nat. Chem. Biol. 16, 988–996 (2020).
Lu, G. J. et al. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Nat. Mater. 17, 456–463 (2018).
Shapiro, M. G. et al. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nat. Chem. 6, 629–634 (2014).
Wang, Y., Roose, B. W., Palovcak, E. J., Carnevale, V. & Dmochowski, I. J. A genetically encoded β-lactamase reporter for ultrasensitive 129Xe NMR in mammalian cells. Angew. Chem. Int. Ed. 55, 8984–8987 (2016).
Lu, G. J. et al. Genetically encodable contrast agents for optical coherence tomography. ACS Nano 14, 7823–7831 (2020).
Farhadi, A. et al. Genetically encoded phase contrast agents for digital holographic microscopy. Nano Lett. 20, 8127–8134 (2020).
Wu, D. et al. Genetically encoded nanostructures enable acoustic manipulation of engineered cells. Preprint at bioRxiv https://doi.org/10.1101/691105 (2019).
Bar-Zion, A. et al. Acoustically detonated biomolecules for genetically encodable inertial cavitation. Preprint at bioRxiv https://doi.org/10.1101/620567 (2019).
Gilbert, C. & Ellis, T. Biological engineered living materials: growing functional materials with genetically programmable properties. ACS Synth. Biol. 8, 1–15 (2019).
Huang, P.-S., Boyken, S. E. & Baker, D. The coming of age of de novo protein design. Nature 537, 320–327 (2016).
Yang, K. K., Wu, Z. & Arnold, F. H. Machine-learning-guided directed evolution for protein engineering. Nat. Methods 16, 687–694 (2019).
Lang, K. & Chin, J. W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 114, 4764–4806 (2014).
Gilad, A. A. & Shapiro, M. G. Molecular imaging in synthetic biology, and synthetic biology in molecular imaging. Mol. Imaging Biol. 19, 373–378 (2017).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Bracha, D., Walls, M. T. & Brangwynne, C. P. Probing and engineering liquid-phase organelles. Nat. Biotechnol. 37, 1435–1445 (2019).
Palmer, A. E., Qin, Y., Park, J. G. & McCombs, J. E. Design and application of genetically encoded biosensors. Trends Biotechnol. 29, 144–152 (2011).
Szablowski, J. O., Bar-Zion, A. & Shapiro, M. G. Achieving spatial and molecular specificity with ultrasound-targeted biomolecular nanotherapeutics. Acc. Chem. Res. 52, 2427–2434 (2019).
Suetens, P. Fundamentals of Medical Imaging (Cambridge Univ. Press, 2017).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Luís Dean-Ben, X. & Razansky, D. Localization optoacoustic tomography. Light Sci. Appl. 7, 18004 (2018).
Seeger, M. et al. Pushing the boundaries of optoacoustic microscopy by total impulse response characterization. Nat. Commun. 11, 2910 (2020).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
We are grateful to members of the Shapiro and Westmeyer laboratories for helpful discussions. Relevant research in the Shapiro laboratory was supported by the National Institutes of Health (grant nos. R01EB018975 and U54CA199090), the Human Frontier Science Program (RGP0050/2016), the Heritage Medical Research Institute, the Packard Foundation, the Pew Charitable Trust, the Sontag Foundation, the Dana Foundation and the Burroughs Wellcome Fund. A.F. was supported by an NSERC graduate fellowship. Relevant research in the Westmeyer laboratory was supported by the European Research Council under grant agreement nos. ERC-StG 311552 and ERC-COG 865710, the Deutsche Forschungsgemeinschaft through the TUM International Graduate School of Science and Engineering and the Federation of European Biochemical Societies.
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farhadi, A., Sigmund, F., Westmeyer, G.G. et al. Genetically encodable materials for non-invasive biological imaging. Nat. Mater. 20, 585–592 (2021). https://doi.org/10.1038/s41563-020-00883-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-00883-3
This article is cited by
-
Engineering water exchange is a safe and effective method for magnetic resonance imaging in diverse cell types
Journal of Biological Engineering (2024)
-
Engineered serum markers for non-invasive monitoring of gene expression in the brain
Nature Biotechnology (2024)
-
Wireless agents for brain recording and stimulation modalities
Bioelectronic Medicine (2023)
-
In-vivo programmable acoustic manipulation of genetically engineered bacteria
Nature Communications (2023)
-
In vivo imaging of mitochondrial DNA mutations using an integrated nano Cas12a sensor
Nature Communications (2023)