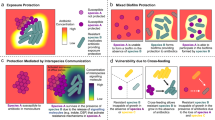
Abstract
Tackling antibiotic resistance necessitates deep understanding of how resource competition within and between species modulates the fitness of resistant microbes. Recent advances in ecological coexistence theory offer a powerful framework to probe the mechanisms regulating intra- and interspecific competition, but the significance of this body of theory to the problem of antibiotic resistance has been largely overlooked. In this Perspective, we draw on emerging ecological theory to illustrate how changes in resource niche overlap can be equally important as changes in competitive ability for understanding costs of resistance and the persistence of resistant pathogens in microbial communities. We then show how different temporal patterns of resource and antibiotic supply, alongside trade-offs in competitive ability at high and low resource concentrations, can have diametrically opposing consequences for the coexistence and exclusion of resistant and susceptible strains. These insights highlight numerous opportunities for innovative experimental and theoretical research into the ecological dimensions of antibiotic resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levy, S. B. & Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10, S122–S129 (2004).
Raymond, B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol. Appl. 12, 1079–1091 (2019).
Perron, G. G., Inglis, R. F., Pennings, P. S. & Cobey, S. Fighting microbial drug resistance: a primer on the role of evolutionary biology in public health. Evol. Appl. 8, 211–222 (2015).
Andersen, S. B., Shapiro, B. J., Vandenbroucke-Grauls, C. & de Vos, M. G. J. Microbial evolutionary medicine: from theory to clinical practice. Lancet Infect. Dis. 19, e273–e283 (2019).
Gilbert, J. A. & Lynch, S. V. Community ecology as a framework for human microbiome research. Nat. Med. 25, 884–889 (2019).
Huijben, S., Chan, B. H. K., Nelson, W. A. & Read, A. F. The impact of within-host ecology on the fitness of a drug-resistant parasite. Evol. Med. Public Health 2018, 127–137 (2018).
Klümper, U. et al. Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. ISME J. 13, 2927–2937 (2019).
Andersson, D. I. & Levin, B. R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2, 489–493 (1999).
Hall, A. R., Angst, D. C., Schiessl, K. T. & Ackermann, M. Costs of antibiotic resistance - separating trait effects and selective effects. Evol. Appl. 8, 261–272 (2015).
Lehtinen, S. et al. Evolution of antibiotic resistance is linked to any genetic mechanism affecting bacterial duration of carriage. Proc. Natl Acad. Sci. USA 114, 1075–1080 (2017).
Colijn, C. et al. What is the mechanism for persistent coexistence of drug-susceptible and drug-resistant strains of Streptococcus pneumoniae? J. R. Soc. Interface 7, 905–919 (2010).
Blanquart, F., Lehtinen, S. & Fraser, C. An evolutionary model to predict the frequency of antibiotic resistance under seasonal antibiotic use, and an application to streptococcus pneumoniae. Proc. R. Soc. B 284, 20170679 (2017).
Holmes, A. H. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387, 176–187 (2016).
Blanquart, F. Evolutionary epidemiology models to predict the dynamics of antibiotic resistance. Evol. Appl. 12, 365–383 (2019).
Davies, N. G., Flasche, S., Jit, M. & Atkins, K. E. Within-host dynamics shape antibiotic resistance in commensal bacteria. Nat. Ecol. Evol. 3, 440–449 (2019).
Melnyk, A. H., Wong, A. & Kassen, R. The fitness costs of antibiotic resistance mutations. Evol. Appl. 8, 273–283 (2015).
Bjourkman, J., Nagaev, I., Berg, O. G., Hughes, D. & Andersson, D. I. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287, 1479–1482 (2000).
Petersen, A., Aarestrup, F. M. & Olsen, J. E. The in vitro fitness cost of antimicrobial resistance in Escherichia coli varies with the growth conditions. FEMS Microbiol. Lett. 299, 53–59 (2009).
Paulander, W., Maisnier-Patin, S. & Andersson, D. I. The fitness cost of streptomycin resistance depends on rpsL mutation, carbon source and RpoS (σS). Genetics 183, 539–546 (2009).
Hall, A. R., Iles, J. C. & MacLean, R. C. The fitness cost of rifampicin resistance in Pseudomonas aeruginosa depends on demand for RNA polymerase. Genetics 187, 817–822 (2011).
Trindade, S., Sousa, A. & Gordo, I. Antibiotic resistance and stress in the light of Fisher’s model. Evolution 66, 3815–3824 (2012).
Tilman, D. Resource Competition and Community Structure (Princeton Univ. Press, 1982).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000).
Chase, J. & Leibold, M. Ecological Niches: Linking Classical and Contemporary Approaches (Univ. Chicago Press, 2003).
Adler, P. B., Hillerislambers, J. & Levine, J. M. A niche for neutrality. Ecol. Lett. 10, 95–104 (2007).
HilleRisLambers, J., Adler, P. B., Harpole, W., Levine, J. M. & Mayfield, M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 43, 227–248 (2012).
Letten, A. D., Ke, P.-J. & Fukami, T. Linking modern coexistence theory and contemporary niche theory. Ecol. Monogr. 87, 161–177 (2017).
Barabás, G., D’Andrea, R. & Stump, S. M. Chesson’s coexistence theory. Ecol. Monogr. 88, 277–303 (2018).
Chesson, P. Updates on mechanisms of maintenance of species diversity. J. Ecol. 106, 1773–1794 (2018).
Ellner, S. P., Snyder, R. E., Adler, P. B. & Hooker, G. An expanded modern coexistence theory for empirical applications. Ecol. Lett. 22, 3–18 (2019).
Chesson, P. Multispecies competition in variable environments. Theor. Popul. Biol. 45, 227–276 (1994).
Adler, P. B., HilleRisLambers, J., Kyriakidis, P. C., Guan, Q. & Levine, J. M. Climate variability has a stabilizing effect on the coexistence of prairie grasses. Proc. Natl Acad. Sci. USA 103, 12793–12798 (2006).
Yuan, C. & Chesson, P. The relative importance of relative nonlinearity and the storage effect in the lottery model. Theor. Popul. Biol. 105, 39–52 (2015).
Wale, N., Sim, D. G. & Read, A. F. A nutrient mediates intraspecific competition between rodent malaria parasites in vivo. Proc. R. Soc. B 284, 20171067 (2017).
Pereira, F. C. & Berry, D. Microbial nutrient niches in the gut. Environ. Microbiol. 19, 1366–1378 (2017).
Hester, E. R., Jetten, M. S. M., Welte, C. U. & Lücker, S. Metabolic overlap in environmentally diverse microbial communities. Front. Genet. 10, 989 (2019).
Smith, V. H. & Holt, R. D. Resource competition and within-host disease dynamics. Trends Ecol. Evol. 11, 386–389 (1996).
Hurtado, P. J., Hall, S. R. & Ellner, S. P. Infectious disease in consumer populations: dynamic consequences of resource-mediated transmission and infectiousness. Theor. Ecol. 7, 163–179 (2014).
Cressler, C. E., Nelson, W. A., Day, T. & McCauley, E. Disentangling the interaction among host resources, the immune system and pathogens. Ecol. Lett. 17, 284–293 (2014).
Smith, V. H., Holt, R. D., Smith, M. S., Niu, Y. & Barfield, M. Resources, mortality, and disease ecology: importance of positive feedbacks between host growth rate and pathogen dynamics. Isr. J. Ecol. Evol. 61, 37–49 (2015).
Alonso, A. et al. Overexpression of the multidrug efflux pump SmeDEF impairs Stenotrophomonas maltophilia physiology. J. Antimicrob. Chemother. 53, 432–434 (2004).
Perkins, A. E. & Nicholson, W. L. Uncovering new metabolic capabilities of Bacillus subtilis using phenotype profiling of rifampin-resistant rpoB mutants. J. Bacteriol. 190, 807–814 (2008).
Martínez, J. L. & Rojo, F. Metabolic regulation of antibiotic resistance. FEMS Microbiol. Rev. 35, 768–789 (2011).
Zampieri, M. et al. Metabolic constraints on the evolution of antibiotic resistance. Mol. Syst. Biol. 13, 917 (2017).
Dunphy, L. J., Yen, P. & Papin, J. A. Integrated experimental and computational analyses reveal differential metabolic functionality in antibiotic-resistant Pseudomonas aeruginosa. Cell Syst. 8, 3-14.e3 (2019).
Webber, M. A. & Piddock, L. J. V. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 51, 9–11 (2003).
Fitzsimmons, J. M., Schoustra, S. E., Kerr, J. T. & Kassen, R. Population consequences of mutational events: effects of antibiotic resistance on the r/K trade-off. Evol. Ecol. 24, 227–236 (2010).
Baltrus, D. A. Exploring the costs of horizontal gene transfer. Trends Ecol. Evol. 28, 489–495 (2013).
San Millan, A. & MacLean, R. C. Fitness costs of plasmids: a limit to plasmid transmission. Microbiol. Spectrum 5, 65–79 (2017).
Dennis, J. J. The evolution of IncP catabolic plasmids. Curr. Opin. Biotechnol. 16, 291–298 (2005).
Shintani, M. et al. Response of the Pseudomonas host chromosomal transcriptome to carriage of the IncP-7 plasmid pCAR1. Environ. Microbiol. 12, 1413–1426 (2009).
San Millan, A. et al. Integrative analysis of fitness and metabolic effects of plasmids in Pseudomonas aeruginosa PAO1. ISME J. 12, 3014–3024 (2018).
Schlüter, A. et al. The 64508 bp IncP-1β antibiotic multiresistance plasmid pB10 isolated from a waste-water treatment plant provides evidence for recombination between members of different branches of the IncP-1β group. Microbiology 149, 3139–3153 (2003).
Chen, K. et al. Comparison of four Comamonas catabolic plasmids reveals the evolution of pBHB to catabolize haloaromatics. Appl. Environ. Microbiol. 82, 1401–1411 (2016).
Douglas, A. E. Fundamentals of Microbiome Science: How Microbes Shape Animal Biology (Princeton Univ. Press, 2018).
Ibrahim, K. H., Gunderson, B. W., Hermsen, E. D., Hovde, L. B. & Rotschafer, J. C. Pharmacodynamics of pulse dosing versus standard dosing: in vitro metronidazole activity against Bacteroides fragilis and Bacteroides thetaiotaomicron. Antimicrob. Agents Chemother. 48, 4195–4199 (2004).
Peña-Miller, R., Lähnemann, D., Schulenburg, H., Ackermann, M. & Beardmore, R. Selecting against antibiotic-resistant pathogens: optimal treatments in the presence of commensal bacteria. Bull. Math. Biol. 74, 908–934 (2012).
Lin, W.-H. & Kussell, E. Complex interplay of physiology and selection in the emergence of antibiotic resistance. Curr. Biol. 26, 1486–1493 (2016).
Bauer, M., Graf, I. R., Ngampruetikorn, V., Stephens, G. J. & Frey, E. Exploiting ecology in drug pulse sequences in favour of population reduction. PLoS Comput. Biol. 13, e1005747 (2017).
Baker, C. M., Ferrari, M. J. & Shea, K. Beyond dose: pulsed antibiotic treatment schedules can maintain individual benefit while reducing resistance. Sci. Rep. 8, 5866 (2018).
Nev, O. A., Jepson, A., Beardmore, R. E. & Gudelj, I. Predicting community dynamics of antibiotic-sensitive and -resistant species in fluctuating environments. J. R. Soc. Interface 17, 20190776 (2020).
Kouyos, R. D. et al. The path of least resistance: aggressive or moderate treatment? Proc. R. Soc. B 281, 20140566 (2014).
Day, T. & Read, A. F. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLoS Comput. Biol. 12, e1004689 (2016).
Alexander, H. K. & MacLean, R. C. Stochastic bacterial population dynamics restrict the establishment of antibiotic resistance from single cells. Proc. Natl Acad. Sci. USA 117, 19455–19464 (2020).
Zarrinpar, A., Chaix, A., Yooseph, S. & Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 (2014).
Thaiss, C. A., Zeevi, D., Levy, M., Segal, E. & Elinav, E. A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes 6, 137–142 (2015).
Kaczmarek, J. L., Thompson, S. V. & Holscher, H. D. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr. Rev. 75, 673–682 (2017).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14 (2009).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Venable, E. B. et al. Effects of feeding management on the equine cecal microbiota. J. Equine Vet. Sci. 49, 113–121 (2017).
Parris, D. J., Morgan, M. M. & Stewart, F. J. Feeding rapidly alters microbiome composition and gene transcription in the clownfish gut. Appl. Environ. Microbiol. 85, e02479-18 (2019).
Chesson, P. & Huntly, N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am. Nat. 150, 519–553 (1997).
Chesson, P. Quantifying and testing coexistence mechanisms arising from recruitment fluctuations. Theor. Popul. Biol. 64, 345–357 (2003).
Watson, S. P., Clements, M. O. & Foster, S. J. Characterization of the starvation-survival response of Staphylococcus aureus. J. Bacteriol. 180, 1750–1758 (1998).
Grover, J. Resource Competition Vol. 19 (Springer Science & Business Media, 1997).
Letten, A. D., Dhami, M. K., Ke, P.-J. & Fukami, T. Species coexistence through simultaneous fluctuation-dependent mechanisms. Proc. Natl Acad. Sci. USA 115, 6745–6750 (2018).
Levison, M. E. & Levison, J. H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 23, 791–815 (2009).
Maharjan, R. & Ferenci, T. The fitness costs and benefits of antibiotic resistance in drug-free microenvironments encountered in the human body. Environ. Microbiol. Rep. 9, 635–641 (2017).
Silva, A. S. et al. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res. 72, 6362–6370 (2012).
Song, T. et al. Fitness costs of rifampicin resistance in Mycobacterium tuberculosis are amplified under conditions of nutrient starvation and compensated by mutation in the β subunit of RNA polymerase. Mol. Microbiol. 91, 1106–1119 (2014).
Hart, S. P., Schreiber, S. J. & Levine, J. M. How variation between individuals affects species coexistence. Ecol. Lett. 19, 825–838 (2016).
Schreiber, S. J., Levine, J. M., Godoy, O., Kraft, N. J. & Hart, S. P. Does deterministic coexistence theory matter in a finite world? Insights from serpentine annual plants. Preprint at bioRxiv https://doi.org/10.1101/290882 (2020).
Data from the ECDC Surveillance Atlas - Antimicrobial Resistance (European Centre for Disease Prevention and Control, 2020); http://go.nature.com/3oLrjOG
Matteo, M. J., Granados, G., Olmos, M., Wonaga, A. & Catalano, M. Helicobacter pylori amoxicillin heteroresistance due to point mutations in PBP-1A in isogenic isolates. J. Antimicrob. Chemother. 61, 474–477 (2008).
Mongkolrattanothai, K. et al. Simultaneous carriage of multiple genotypes of Staphylococcus aureus in children. J. Med. Microbiol. 60, 317–322 (2011).
Folkvardsen, D. B. et al. Rifampin heteroresistance in Mycobacterium tuberculosis cultures as detected by phenotypic and genotypic drug susceptibility test methods. J. Clin. Microbiol. 51, 4220–4222 (2013).
Kamng’ona, A. W. et al. High multiple carriage and emergence of Streptococcus pneumoniae vaccine serotype variants in Malawian children. BMC Infect. Dis. 15, 234 (2015).
Andersson, D. I., Nicoloff, H. & Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat. Rev. Microbiol. 17, 479–496 (2019).
Chesson, P. & Kuang, J. J. The interaction between predation and competition. Nature 456, 235–238 (2008).
Gonze, D., Coyte, K. Z., Lahti, L. & Faust, K. Microbial communities as dynamical systems. Curr. Opin. Microbiol. 44, 41–49 (2018).
Godoy, O., Kraft, N. J. B. & Levine, J. M. Phylogenetic relatedness and the determinants of competitive outcomes. Ecol. Lett. 17, 836–844 (2014).
Kraft, N. J. B., Godoy, O. & Levine, J. M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 112, 797–802 (2015).
Hart, S. P., Freckleton, R. P. & Levine, J. M. How to quantify competitive ability. J. Ecol. 106, 1902–1909 (2018).
Hallinen, K. M., Karslake, J. & Wood, K. B. Delayed antibiotic exposure induces population collapse in enterococcal communities with drug-resistant subpopulations. eLife 9, e52813 (2020).
Gould, A. L. et al. Microbiome interactions shape host fitness. Proc. Natl Acad. Sci. USA 115, E11951–E11960 (2018).
Torres-Barceló, C. & Hochberg, M. E. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 24, 249–256 (2016).
Burmeister, A. R. et al. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl Acad. Sci. USA 117, 11207–11216 (2020).
Butler, S. & O’Dwyer, J. P. Stability criteria for complex microbial communities. Nat. Commun. 9, 2970 (2018).
Estrela, S. & Brown, S. P. Community interactions and spatial structure shape selection on antibiotic resistant lineages. PLoS Comput. Biol. 14, e1006179 (2018).
Acknowledgements
We thank D. McLeod, S. Bonhoeffer and the Pathogen Ecology group at ETH for thoughtful discussions and comments on an earlier draft of this manuscript. This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skodowska-Curie grant agreement no. 750779.
Author information
Authors and Affiliations
Contributions
A.D.L., A.R.H. and J.M.L. conceived the study. A.D.L. performed simulations and analysis. A.D.L., A.R.H. and J.M.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Ecology & Evolution thanks Nina Wale and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Information 1–4.
Rights and permissions
About this article
Cite this article
Letten, A.D., Hall, A.R. & Levine, J.M. Using ecological coexistence theory to understand antibiotic resistance and microbial competition. Nat Ecol Evol 5, 431–441 (2021). https://doi.org/10.1038/s41559-020-01385-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-01385-w
This article is cited by
-
Lose-lose consequences of bacterial community-driven invasions in soil
Microbiome (2024)
-
CRISPR-Cas-based identification of a sialylated human milk oligosaccharides utilization cluster in the infant gut commensal Bacteroides dorei
Nature Communications (2024)
-
Horizontal gene transfer is predicted to overcome the diversity limit of competing microbial species
Nature Communications (2024)
-
Differential carbon utilization enables co-existence of recently speciated Campylobacteraceae in the cow rumen epithelial microbiome
Nature Microbiology (2023)
-
Antibiotic resistance in the environment
Nature Reviews Microbiology (2022)