Abstract
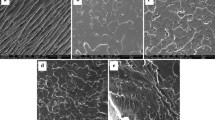
This study aims to evaluate the effects of poly(2-ethyl-oxazoline) (PEOx) on the thermal properties, wettability, and optical properties of poly(lactic acid) (PLA). The PLA/PEOx blends (PEOx loading: 5 to 20 wt%) were prepared using the solvent casting method. Good interaction between PLA and PEOx was confirmed by Fourier transform infrared spectroscopy (FTIR) and work of adhesion (determined from dynamic contact angle). The transparency of PLA was improved by the addition of PEOx. X-ray diffraction (XRD) and differential scanning calorimetry (DSC) results proved that the PEOx induces the β-crystals formation of PLA. The higher the loading of PEOx is, the higher the intensity of the β-crystals formation. The transparent PLA/PEOx blends are suitable for packaging applications (both non-food and food packaging).






Similar content being viewed by others
References
Niaounakis M (2019) Recycling of biopolymers—the patent perspective. Eur Polym J 114:464–475. https://doi.org/10.1016/j.eurpolymj.2019.02.027
Chen GQ, Martin KP (2012) Plastics derived from biological sources: present and future: a technical and environmental review. Chem Rev 112:2082–2099. https://doi.org/10.1021/cr200162d
Ruggero F, Gori R, Lubello C (2019) Methodologies to assess biodegradation of bioplastics during aerobic composting and anaerobic digestion: a review. Waste Manag Res 37:959–975. https://doi.org/10.1177/0734242X19854127
Castro-Aguirre E, Iñiguez-Franco F, Samsudin H et al (2016) Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev 107:333–366. https://doi.org/10.1016/j.addr.2016.03.010
Aamer AS, Fariha H, Abdul H et al (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Jamshidian M, Tehrany EA, Imran M et al (2010) Polylactic acid: production, applications, nanocomposites, and release studies. Compr Rev Food Sci Food Saf 9:552–571. https://doi.org/10.1111/j.1541-4337.2010.00126.x
Tham WL, Poh BT, Mohd Ishak ZA et al (2014) Thermal behaviors and mechanical properties of halloysite nanotube-reinforced poly(lactic acid) nanocomposites. J Therm Anal Calorim 118:1639–1647. https://doi.org/10.1007/s10973-014-4062-2
Nofar M, Sacligil D, Carreau PJ et al (2019) Poly(lactic acid) blends: processing, properties and applications. Int J Biol Macromol 125:307–360. https://doi.org/10.1016/j.ijbiomac.2018.12.002
Wang M, Wu Y, Li YD et al (2017) Progress in toughening poly(lactic acid) with renewable polymers. Polym Rev 57:557–593. https://doi.org/10.1080/15583724.2017.1287726
Jin FL, Hu RR, Park SJ (2019) Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: a review. Compos B Eng 164:287–296. https://doi.org/10.1016/j.compositesb.2018.10.078
Saeidlou S, Huneault MA, Li H et al (2012) Poly(lactic acid) crystallization. Prog Polym Sci 37:1657–1677. https://doi.org/10.1016/j.progpolymsci.2012.07.005
Kaseem M, Ko YG (2017) Melt flow behavior and processability of polylactic acid/polystyrene (PLA/PS) polymer blends. J Polym Environ 25:994–998. https://doi.org/10.1007/s10924-016-0873-5
Leu YY, Chow WS (2011) Kinetics of water absorption and thermal properties of poly(lactic acid)/organomontmorillonite/poly(ethylene glycol) nanocomposites. J Vinyl Addit Technol 17:40–47. https://doi.org/10.1002/vnl.20259
Zhou H, Zhao M, Qu Z et al (2018) Thermal and rheological properties of poly(lactic acid)/low-density polyethylene blends and their supercritical CO2 foaming behavior. J Polym Environ 26:3564–3573. https://doi.org/10.1007/s10924-018-1240-5
Jia W, Gong RH, Soutis C, Hogg PJ (2014) Biodegradable fibre reinforced composites composed of polylactic acid and polybutylene succinate. Plast, Rubber Compos 43:82–88. https://doi.org/10.1179/1743289813Y.0000000070
Iglesias Montes ML, D’amico DA, Manfredi LB, Cyras VP (2019) Effect of natural glyceryl tributyrate as plasticizer and compatibilizer on the performance of bio-based polylactic acid/poly(3-hydroxybutyrate) blends. J Polym Environ 27:1429–1438. https://doi.org/10.1007/s10924-019-01425-y
Torres-Huerta AM, Palma-Ramírez D, Domínguez-Crespo MA et al (2014) Comparative assessment of miscibility and degradability on PET/PLA and PET/chitosan blends. Eur Polym J 61:285–299. https://doi.org/10.1016/j.eurpolymj.2014.10.016
Samthong C, Deetuam C, Yamaguchi M et al (2016) Effects of size and shape of dispersed poly(butylene terephthalate) on isothermal crystallization kinetics and morphology of poly(lactic acid) blends. Polym Eng Sci 56:258–268. https://doi.org/10.1002/pen.24246
Teoh EL, Chow WS (2017) Transparency, ultraviolet transmittance, and miscibility of poly(lactic acid)/poly(methyl methacrylate) blends. J Elastomers Plast 50:596–610. https://doi.org/10.1177/0095244317743067
Qu Z, Yin D, Zhou H, Wang X, Zhao S (2019) Cellular morphology evolution in nanocellular poly (lactic acid)/thermoplastic polyurethane blending foams in the presence of supercritical N2. Eur Polym J 116:291–301. https://doi.org/10.1016/j.eurpolymj.2019.03.046
Lu X, Huang J, Kang B, Yuan T, Qu J (2019) Bio-based poly (lactic acid)/high-density polyethylene blends as shape-stabilized phase change material for thermal energy storage applications. Sol Energy Mater Sol Cells 192:170–178. https://doi.org/10.1016/j.solmat.2018.12.036
Zhao M, Ding X, Mi J, Zhou H, Wang X (2017) Role of high-density polyethylene in the crystallization behaviors, rheological property, and supercritical CO2 foaming of poly (lactic acid). Polym Degrad Stab 146:277–286. https://doi.org/10.1016/j.polymdegradstab.2017.11.003
Jašo V, Glenn G, Artur Klamczynski, Petrović ZS (2015) Biodegradability study of polylactic acid/thermoplastic polyurethane blends. Polym Test 47:1–3. https://doi.org/10.1016/j.polymertesting.2015.07.011
Cocca M, Androsch R, Righetti MC, Malinconico M, Lorenzo MLD (2014) Conformationally disordered crystals and their influence on material properties: the cases of isotactic polypropylene, isotactic poly(1-butene), and poly(l-lactic acid). J Mol Struct 1078:114–132. https://doi.org/10.1016/j.molstruc.2014.02.038
Xu R, Xie J, Lei C (2017) Influence of melt-draw ratio on the crystalline behaviour of a polylactic acid cast film with a chi structure. RSC Adv 7:39914–39921. https://doi.org/10.1039/c7ra05422j
Singh NK, Singh SK, Dash D, Gonugunta P, Misra M, Maiti P (2013) CNT induced β-phase in polylactide: unique crystallization, biodegradation, and biocompatibility. J Phys Chem C 117:10163–10174. https://doi.org/10.1021/jp4009042
Su L, Zou J, Dong S, Hao N, Xu H (2017) Influence of different β-nucleation agents on poly(l-lactic acid): structure, morphology, and dynamic mechanical behavior. RSC Adv 7:55364–55370. https://doi.org/10.1039/C7RA10550A
Saha D, Samal SK, Biswal M, Mohanty S, Nayak SK (2019) Preparation and characterization of poly(lactic acid)/poly(ethylene oxide) blend film: effects of poly(ethylene oxide) and poly(ethylene glycol) on the properties. Polym Int 68:164–172. https://doi.org/10.1002/pi.5718
Isasi JR, Meaurio E, Cesteros C et al (1996) Miscibility and specific interactions in blends of poly(2-ethyl-2-oxazoline) with hydroxylated polymethacrylates. Macromol Chem Phys 197:641–649. https://doi.org/10.1002/macp.1996.021970219
Maldonado-Santoyo M, Ortíz-Estrada C, Luna-Bárcenas G et al (2014) Miscibility behavior and hydrogen bonding in blends of poly(vinyl phenyl ketone hydrogenated) and poly(2-ethyl-2-oxazoline). J Polym Sci, Part B: Polym Phys 42:636–645. https://doi.org/10.1002/polb.10758
Zhang C, Liu S, Tan L et al (2015) Star-shaped poly(2-methyl-2-oxazoline)-based films: rapid preparation and effects of polymer architecture on antifouling properties. J Mater Chem B 3:5615–5628. https://doi.org/10.1039/C5TB00732A
Schneider M, Tang Z, Richter M et al (2016) Patterned polypeptoid brushes. Macromol Biosci 16:75–81. https://doi.org/10.1002/mabi.201500314
Popelka A, Kronek J, Novák I et al (2014) Surface modification of low-density polyethylene with poly(2-ethyl-2-oxazoline) using a low-pressure plasma treatment. Vacuum 100:53–56. https://doi.org/10.1016/j.vacuum.2013.07.016
Li D, Neumann AW (1992) Equation of state for interfacial tensions of solid-liquid systems. Adv Colloid Interface Sci 39:299–345. https://doi.org/10.1016/0001-8686(92)80064-5
Roura P, Fort J (2004) Local thermodynamic derivation of Young’s equation. J Colloid Interface Sci 272:420–429. https://doi.org/10.1016/j.jcis.2004.01.028
Stöckelhuber KW, Das A, Jurk R, Heinrich G (2010) Contribution of physico-chemical properties of interfaces on dispersibility, adhesion and flocculation of filler particles in rubber. Polymer 51:1954–1963. https://doi.org/10.1016/j.polymer.2010.03.013
Chapple S, Anandjiwala R, Ray SS (2013) Mechanical, thermal, and fire properties of polylactide/starch blend/clay composites. J Therm Anal Calorim 113:703–712. https://doi.org/10.1007/s10973-012-2776-6
Zhang T, Yu Q, Wang J et al (2018) Design and fabrication of a renewable and highly transparent multilayer coating on poly(lactic acid) film capable of UV-shielding and antifogging. Ind Eng Chem Res 57:4577–4584. https://doi.org/10.1021/acs.iecr.8b00120
Ma Y, Bao J, Zhang Y et al (2019) Mammalian near-infrared image vision through injectable and self-powered retinal nanoantennae. Cell 177:243–255. https://doi.org/10.1016/j.cell.2019.01.038
Macgregor-Ramiasa MN, Cavallaro AA, Vasilev K (2015) Properties and reactivity of polyoxazoline plasma polymer films. J Mater Chem B 3:6327–6337. https://doi.org/10.1039/C5TB00901D
Macgregor-Ramiasa MN, Cavallaro AA, Mierczynska A et al (2015) Plasma polymerised polyoxazoline thin films for biomedical applications. Chem Commun 51:4279–4282. https://doi.org/10.1039/C5CC00260E
Newberry RW, Raines RT (2017) The n → π* interaction. Acc Chem Res 50:1838–1846. https://doi.org/10.1021/acs.accounts.7b00121
Choudhary A, Gandla D, Krow GR, Raines RT (2009) Nature of amide carbonyl–carbonyl interactions in proteins. J Am Chem Soc 131:7244–7246. https://doi.org/10.1021/ja901188y
Oliveira JE, Moraes EA, Marconcini JM et al (2013) Properties of poly(lactic acid) and poly(ethylene oxide) solvent polymer mixtures and nanofibers made by solution blow spinning. J Appl Polym Sci 129:3672–3681. https://doi.org/10.1002/app.39061
Hongen T, Taniguchi T, Nomura S, Kadokawa J, Monde K (2014) In depth study on solution-state structure of poly(lactic acid) by vibrational circular dichroism. Macromolecules 47:5313–5319. https://doi.org/10.1021/ma501020s
Siročić AP, Hrnjak-Murgić Z, Jelenčić J (2013) The surface energy as an indicator of miscibility of SAN/EDPM polymer blends. J Adhes Sci Technol 27:2615–2628. https://doi.org/10.1080/01694243.2013.796279
Kurusu RS, Demarquette NR (2017) Surface properties evolution in electrospun polymer blends by segregation of hydrophilic or amphiphilic molecules. Eur Polym J 89:129–137. https://doi.org/10.1016/j.eurpolymj.2017.02.016
Vrsaljko D, Grčić I, Guyon C et al (2016) Designing hydrophobicity of the PLA polymer blend surfaces by ICP etching. Plasma Process Polym 13:869–878. https://doi.org/10.1002/ppap.201500218
Khoshkava V, Kamal MR (2013) Effect of surface energy on dispersion and mechanical properties of polymer/nanocrystalline cellulose nanocomposites. Biomacromol 14:3155–3163. https://doi.org/10.1021/bm400784j
Singh A, Naskar AK, Barden J et al (2011) Terpolymers from lactide and bisphenol a derivatives: scale up, properties, and blends. Appl Polym Sci 122:2520–2528. https://doi.org/10.1002/app.33789
Hári J, Horváth F, Móczó J et al (2017) Competitive interactions, structure and properties in polymer/layered silicate nanocomposites. Express Polym Lett 11:479–492. https://doi.org/10.3144/expresspolymlett.2017.45
Montes-Zavala I, Castrejón-González EO, Sánchez-Balderas G et al (2020) Effect of H bonds on thermal behavior and cohesion in polylactic acid nanocomposites and nitrogen-doped carbon nanotubes. J Mater Sci 55:3354–3368. https://doi.org/10.1007/s10853-019-04245-6
Guerrica-Echevarrı́a G, Eguiazábal JI, Nazábal J (2000) Interfacial tension as a parameter to characterize the miscibility level of polymer blends. Polym Test 19:849–854. https://doi.org/10.1016/S0142-9418(99)00055-0
Nematollahi M, Jalali Arani A, Modarress H (2019) Effect of nanoparticle localization on the rheology, morphology and toughness of nanocomposites based on poly(lactic acid)/natural rubber/nanosilica. Polym Int 68:779–787. https://doi.org/10.1002/pi.5767
Chen J, Cui X, Zhu Y, Jiang W, Sui K (2017) Design of superior conductive polymer composite with precisely controlling carbon nanotubes at the interface of a co-continuous polymer blend via a balance of π-π interactions and dipole-dipole interactions. Carbon 114:441–448. https://doi.org/10.1016/j.carbon.2016.12.048
Jańczuk B, Białopiotrowicz T, Wójcik W (1989) The components of surface tension of liquids and their usefulness in determinations of surface free energy of solids. J Colloid Interface Sci 127:59–66. https://doi.org/10.1016/0021-9797(89)90007-6
Jiang J, Zhu L, Zhu L, Zhu B, Xu Y (2011) Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 27:14180–14187. https://doi.org/10.1021/la202877k
Wang D, Cornelius CJ (2016) Ionomer thermodynamic interrelationships associated with wettability, surface energy, swelling, and water transport. Eur Polym J 85:126–138. https://doi.org/10.1016/j.eurpolymj.2016.10.024
Rigoussen A, Verge P, Raquez JM, Habibi Y, Dubois P (2017) In-depth investigation on the effect and role of cardanol in the compatibilization of PLA/ABS immiscible blends by reactive extrusion. Eur Polym J 93:272–283. https://doi.org/10.1016/j.eurpolymj.2017.06.004
David DJ, Sincock TF (1992) Estimation of miscibility of polymer blends using the solubility parameter concept. Polymer 33:4505–4514. https://doi.org/10.1016/0032-3861(92)90406-M
Jo E, Yeo JG, Kim DK, Oh JS, Hong CK (2014) Preparation of well-controlled porous carbon nanofiber materials by varying the compatibility of polymer blends. Polym Int 63:1471–1477. https://doi.org/10.1002/pi.4645
Choi JH, Lee HY, Towns AD (2010) Dyeing properties of novel azo disperse dyes derived from phthalimide and color fastness on poly(lactic acid) fiber. Fibers Polym 11:199–204. https://doi.org/10.1007/s12221-010-0199-1
Lübtow MM, Haider MS, Kirsch M, Klisch S, Luxenhofer R (2019) Like dissolves like? A comprehensive evaluation of partial solubility parameters to predict polymer-drug compatibility in ultra-high drug loaded polymer micelles. Biomacromol 20:3041–3056. https://doi.org/10.1021/acs.biomac.9b00618
Stefanis E, Panayiotou C (2012) A new expanded solubility parameter approach. Int J Pharm 426:29–43. https://doi.org/10.1016/j.ijpharm.2012.01.001
Aliotta L, Cinelli P, Coltelli MB, Righetti MC, Gazzano M, Lazzeri A (2017) Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PLA). Eur Polym J 93:822–832. https://doi.org/10.1016/j.eurpolymj.2017.04.041
Wang H, Zhang J, Tashiro K (2017) Phase transition mechanism of poly(l-lactic acid) among the α, δ, and β forms on the basis of the reinvestigated crystal structure of the β form. Macromolecules 50:3285–3300. https://doi.org/10.1021/acs.macromol.7b00272
Xie Q, Bao J, Shan G, Bao Y, Pan P (2019) Fractional crystallization kinetics and formation of metastable β-form homocrystals in poly(l-lactic acid)/poly(d-lactic acid) racemic blends induced by precedingly formed stereocomplexes. Macromolecules 52:4655–4665. https://doi.org/10.1021/acs.macromol.9b00644
Restrepo I, Flores P, Rodríguez-Llamazares S (2019) Antibacterial nanocomposite of poly(lactic acid) and ZnO nanoparticles stabilized with poly(vinyl alcohol): thermal and morphological characterization. Polym Plast Technol Eng 58:105–112. https://doi.org/10.1080/03602559.2018.1466168
Tábi T, Sajó IE, Sajó F et al (2010) Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym Lett 4:659–668. https://doi.org/10.3144/expresspolymlett.2010.80
Shen W, Wu W, Liu C et al (2020) Thermal conductivity enhancement of PLA/TPU/BN composites by controlling BN distribution and annealing treatment. Plast, Rubber Compos 49:204–213. https://doi.org/10.1080/14658011.2020.1729657
Rashidi H, Oshani BN, Hejazi I et al (2010) Tuning crystallization and hydrolytic degradation behaviors of poly(lactic acid) by using silver phosphate, zinc oxide and their nano-hybrids. Polym Plast Technol Eng 59:72–82. https://doi.org/10.1080/25740881.2019.1625382
Liu Z, Fu M, Ling F et al (2019) Stereocomplex-type polylactide with bimodal melting temperature distribution: toward desirable melt-processability and thermomechanical performance. Polymer 169:21–28. https://doi.org/10.1016/j.polymer.2019.02.029
He L, Song F, Li D et al (2020) Strong and tough polylactic acid-based composites enabled by simultaneous reinforcement and interfacial compatibilization of microfibrillated cellulose. ACS Sustain Chem Eng 8:1573–1582. https://doi.org/10.1021/acssuschemeng.9b06308
Meng B, Deng J, Liu Q et al (2012) Transparent and ductile poly(lactic acid)/poly(butyl acrylate) (PBA) blends: structure and properties. Eur Polym J 48:127–135. https://doi.org/10.1016/j.eurpolymj.2011.10.009
Cartier L, Okihara T, Ikada Y et al (2000) Epitaxial crystallization and crystalline polymorphism of polylactides. Polymer 41:8909–8919. https://doi.org/10.1016/S0032-3861(00)00234-2
Di Lorenzo ML, Rubino P, Luijkx R et al (2014) Influence of chain structure on crystal polymorphism of poly(lactic acid). Part 1: effect of optical purity of the monomer. Colloid Polym Sci 292:399–409. https://doi.org/10.1007/s00396-013-3081-z
Xie Q, Han L, Shan G et al (2016) Polymorphic crystalline structure and crystal morphology of enantiomeric poly(lactic acid) blends tailored by a self-assemble aryl amide nucleator. ACS Sustain Chem Eng 4:2680–2688. https://doi.org/10.1021/acssuschemeng.6b00191
Puiggali J, Ikada Y, Tsuji H et al (2000) The frustrated structure of poly(L-lactide). Polymer 41:8921–8930. https://doi.org/10.1016/S0032-3861(00)00235-4
Wasanasuk K, Tashiro K (2011) Crystal structure and disorder in poly(l-lactic acid) δ form (α′ form) and the phase transition mechanism to the ordered α form. Polymer 52:6097–6109. https://doi.org/10.1016/j.polymer.2011.10.046
Chen X, Kalish J, Hsu SL (2011) Structure evolution of α′ phase poly(lactic acid). J Polym Sci, Part B: Polym Phys 49:1446–1454. https://doi.org/10.1002/polb.22327
Manafi P, Ghasemi I, Karrabi M, Azizi H, Ehsaninamin P (2014) Effect of graphene nanoplatelets on crystallization kinetics of poly (lactic acid). Soft Mater 12:433–444. https://doi.org/10.1080/1539445X.2014.959598
de Oca HM, Ward IM (2007) Structure and mechanical properties of poly(L-lactic acid) crystals and fibers. J Polym Sci B Polym Phys 45:892–902. https://doi.org/10.1002/polb.21131
Kim Y, Kim JS, Lee SY et al (2020) Exploration of hybrid nanocarbon composite with polylactic acid for packaging applications. Int J Biol Macromol 144:135–142. https://doi.org/10.1016/j.ijbiomac.2019.11.239
Bao J, Chang X, Xie Q et al (2017) Preferential formation of β-form crystals and temperature-dependent polymorphic structure in supramolecular poly(l-lactic acid) bonded by multiple hydrogen bonds. Macromolecules 50:8619–8630. https://doi.org/10.1021/acs.macromol.7b01705
Hughes J, Thomas R, Byun Y, Whiteside S (2012) Improved flexibility of thermally stable poly-lactic acid (PLA). Carbohydr Polym 88:165–172. https://doi.org/10.1016/j.carbpol.2011.11.078
Funding
This work was supported by Universiti Sains Malaysia Research University Grant (Grant Number 8014024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ng, W.K., Chow, W.S. & Ismail, H. Poly(2-ethyl-2-oxazoline) as β-Nucleating Agent for Poly(lactic acid) Blends with High Transparency and Hydrophilicity. J Polym Environ 29, 2650–2659 (2021). https://doi.org/10.1007/s10924-021-02081-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02081-x