South East European International Institute for Sustainable Technologies (SEEIIST)
- 1TERA Foundation, Novara, Italy
- 2SEEIIST Association, Geneva, Switzerland
- 3CERN, Geneva, Switzerland
- 4Department of Physics, University of Oxford, Oxford, United Kingdom
- 5GSI Helmholtzzentrum für Schwerionenforschung, Darmstadt, Germany
- 6Technische Universität Darmstadt, Darmstadt, Germany
- 7HIT, University of Heidelberg, Heidelberg, Germany
- 8Cosylab, Ljubljana, Slovenia
- 9Faculty of Natural Sciences and Mathematics, UKIM, Skopje, North Macedonia
- 10CNAO, Pavia, Italy
- 11Microelectronics & Nanoelectronics, Faculty of Information & Communication Technology, University of Malta, Msida, Malta
- 12Ruprecht-Karls-Universitaet Heidelberg, Heidelberg, Germany
The South East European International Institute for Sustainable Technologies (SEEIIST) was proposed in 2016 at the World Academy of Art and Science, with the objective of building a facility for charged particle cancer therapy for the South Eastern European countries. SEEIIST will offer the world-class research needed to reduce or even revert the brain drain that is causing a shortage of talent and economic losses in South East Europe. There is no particle therapy in South-East Europe in spite of a growing number of cancers being diagnosed. The facility beam time will be shared 50:50 between treating patients and performing research with a wide spectrum of different light ions beyond the presently used protons and carbon ions, which will make the facility unique in the world. SEEIIST Project is presently in a Conceptual to a Design Phase, implemented with the support of the EU and the involvement of CERN and GSI. The next phase of the project realization will include a final technical design for the facility, a structure and a business plan for the organization and the definition of conditions for the site selection.
Introduction
The SEE region consists of the countries that are EU Member States (Bulgaria, Croatia, Greece and Slovenia), as well as of the countries that are aspiring for membership in the near future (Albania, Bosnia and Herzegovina, Kosovo, Montenegro, North Macedonia and Serbia). Due to recent turbulent times in South East Europe, all scientific and economic activities have slowed down. As a consequence, the region also suffered from an extensive brain drain of the young and prosperous scientists. In contrast, the region once featured intensive research and technological development and made significant scientific contributions on the European scale. A prime example of this is the first research nuclear reactor in the former Yugoslavia that was operational already in 1959, only two years after such a research reactor was commissioned in Germany. It is worth mentioning that this region (former Yugoslavia) played an important role as a cofounder of CERN in 19541 as well. The most efficient and effective way to recover this tradition, i.e., to catch up with the EU current excellent research and to revert the brain drain, is to establish a large-scale internationally competitive research infrastructure in the SEE region. To meet this goal, the SEE countries have recently consolidated their forces to set up a large-scale competitive research infrastructure–the South East European International Institute for Sustainable Technologies (SEEIIST2).
Origin of SEEIIST
The idea of SEEIIST was conceived more than 2 years ago, when the Government of Montenegro, led by the Minister of Science, Dr Sanja Damjanovic, initiated the establishment of the SEEIIST Project, originally proposed by Prof. Herwig Schopper, a former Director General of CERN. The initiative was formalized as a regional project once a Declaration of Intent was signed, on October 25, 2017 at a Ministerial meeting at CERN. The signatory parties were Albania, Bosnia and Herzegovina, Bulgaria, Kosovo*3, Montenegro, Serbia, Slovenia and Northern Macedonia. Croatia and Greece took an observer status. Most recently, a SEEIIST Memorandum of Cooperation was signed by six Prime Ministers of the countries of the region (Albania, Bosnia and Herzegovina, Bulgaria, Kosovo, Montenegro and North Macedonia) on July 5, 2019 during the Berlin Process Summit at Poznan, Poland.
In response to this initiative, the EC stated that in order to bring “…our citizens and economies closer together (…) it is determined to strengthen and intensify its engagement at all levels to support the region’s political, economic and social transformation, including through increased assistance.” The statement underlined the ongoing efforts by the EC to bring the SEE countries closer to the EU in terms of its shared values, social cohesion and economic prosperity. Outgoing EC President Juncker, in his 2018 State of the Union Address, and the incoming EC President Von der Leyen, have both stressed the need for intensive cooperation, and Von der Leyen has stated that this would be one of the priorities for the next 5 years.
The overarching objective of the SEEIIST project is to foster regional cooperation in the fields of science, health care, technology, innovation and industry in the spirit of the existing joint research infrastructures successfully implementing the model of ‘Science for Peace,’ such as CERN and SESAME. The project has three main socio-economic objectives: 1) making hadron cancer treatment available to the patients from the SEE region; 2) promoting transnational collaboration between science, technology and industry by bringing together the people from different countries of the region, not only scientists and medical doctors, but also engineers, industrial and administration personnel; 3) providing a common platform to educate talented young people and engineers on the basis of knowledge and technology transfer from European centers, such as CERN and others, and finally mitigating or even reverting the brain drain from the SEE region. This research infrastructure would greatly address the common challenges and needs in the SEE region, triggering, in particular, the sustainable development of economy and social cohesion.
The scope of the SEEIIST is to be an international research infrastructure not only for researchers but also for medical treatment. This implies that all medical infrastructure required will be available at this international center. The Business plan prepared for the SEEIIST@ESFRI application contains this concept as part of the investment. The site of SEEIIST will be sufficiently close to an existing hospital for supplementary medical treatment when necessary.
Joint Research Infrastructure – SEEIIST
The new RI - South East European International Institute for Sustainable Technologies (SEEIIST) will focus on hadron cancer therapy and biomedical research with protons and heavy ions. SEEIIST will thus enable scientists from different countries to work together in the fight against cancer. This particular initiative has been chosen partly because it binds people together against a “common enemy,” but also as an example of cooperation among people in the region. In this regard, SEEIIST’s mission is aligned with the basic concepts behind other large-scale RIs, such as CERN: Science for Peace, Science for Diplomacy and Science for Society. A second reason for placing a hadron facility in the SEE area is the fact that in contrast to Western Europe, no technical provision exists in SEE to treat patients with certain malignant types of tumors with this modality. The selection of a hadron facility over other types of Radiation Therapy (RT), like an X-ray treatment center, or other non-radiological treatment modalities (such as immunotherapy), is motivated by the fact that a particle therapy center is urgently needed to achieve major research advances in pre-clinical physics, pre-clinical radiobiology and medical physics related to cancer treatment, as well as a means to retain the young and talented research human potential in the region.
SEEIIST state-of-the-art RI has already moved from a conceptual to a design phase, thanks to the first financial support from the European Commission. The status of the project was presented to the public at a SEEIIST Kick-off meeting4 ‘Start of the SEEIIST Design Phase,’ held on September 18, 2019 in Budva, Montenegro. The next steps are underway for preparing a defined technical design for the facility, to propose a user’s structure and business plan for the organization and to define the conditions for the site selection. The SEEIIST site selection process is planned to be completed by early 2021, whereas the construction is expected to start in 2023. The first patient is expected to be treated in 2029.
SEEIIST will maintain strong collaboration links with all the relevant particle therapy cancer research groups in Europe, United States, and Japan, it should be noted that there are already links with PTCOG, ESTRO EPTN, and ENLIGHT. Dedicated and specific networking will be initiated with the groups who are currently involved in research in molecular targeting for radioresistant tumors, cancer molecular research, immunotherapy, and the groups that examine the effective antitumor immune response induced by PT. The next ambition for SEEIIST is to become part of the EIT Health Regional Innovation Scheme.
Scientific Case for SEEIIST Facility
Cancer is a critical societal issue. Worldwide, in 2018 alone, 18.1 million cases were diagnosed, 9.6 million people died and 43.8 million people were living with cancer [1, 2]. Currently, it is the second leading cause of death [3], after cardiovascular diseases but recent extrapolations show that it could take over and become the leading cause of death [4]. Demographic drivers of increasing population5 size, life expectancy and aging populations (particularly in higher-income countries), along with progress against many other causes of deaths, imply that the total number of cancer deaths continues to increase. Current projections anticipate an increase with approximately 24.6 million newly diagnosed patients, and 13 million related deaths by 20305, Figure 1 shows the mortality-to-incidence ratio in most of the countries in Europe in 2015. As shown in the figure, in this common fight, some countries struggle more than the others partly because of the lack of advanced diagnostics and treatment equipment. In particular, in the heart of Europe, in its South Eastern (SEE) region, the mortality rates from tumors are up to 40% higher compared to the rest of Europe [4]. Cancer not only has a negative impact on an individual’s health but also comes at a very high cost to the economy. Cancer costs the EU circa €126 billion with health care accounting for €51 billion, productivity losses due to early death estimated at €43 billion, lost working days estimated at €9 billion and informal care estimated at €23 billion [5]. It is for this reason that the European Commission invested €1.6 billion in FP7 and, so far, €1.2 billion in H2020 on cancer research. H2020 policy prioritizes health and wellbeing to be a societal challenge under which cancer research is categorized6. In Horizon Europe, the commission gives the fight against cancer even more priority by considering it to be one of the greatest world challenges and specifically placing the mission against cancer as a top priority in its mission-oriented policy7.
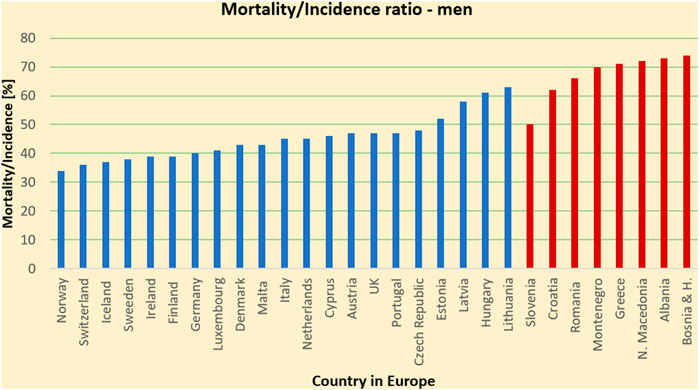
FIGURE 1. Mortality-to-incidence ratio due to all cancers, all ages, man (2018) for SEE and several Western EU countries for comparison. The image highlights a higher outcome with fatality of the cancer patients in the SEE countries compared to some of the EU countries.
Currently over half of the patients diagnosed with cancer undergo radiation therapy (RT), and about 50% of all cured cancer patients have RT as part of their treatment [1, 5]. In this scenario, any significant improvements in RT could have a dramatic impact on patient survival, quality of life and economic costs.
Research and innovation efforts have been currently carried out worldwide to improve the effectiveness of RT. The main goal of advanced radiotherapy treatment is to maximize the damage of ionizing radiation to the tumor cells while minimizing exposure of the surrounding normal tissue and critical organs, to enhance the likelihood of patient cure while the side effects of the treatment are minimized. To achieve this goal, RT has considerably progressed with the development of new technologies and methodologies able to increase the conformity of the dose delivered to deep-seated tumors. While the most frequently used modern RT modalities still rely on high energy (MeV) X-rays, there is a rapidly growing interest in the curative effects of accelerated charged particles, i.e., protons and heavier ions, such as carbon. This so-called particle therapy (PT) can offer superior tumor-dose conformality with a reduced number of treatment fields, compared to conventional X-ray radiation, mainly due to the favourable depth-dose deposition of ions in tissue, presented in Figure 2. However, despite the considerable recent progress of PT, numerous challenges and new opportunities are yet to be addressed to maximize clinical outcome and cost-effectiveness of this advanced RT modality for improved and uniformly accessible healthcare.
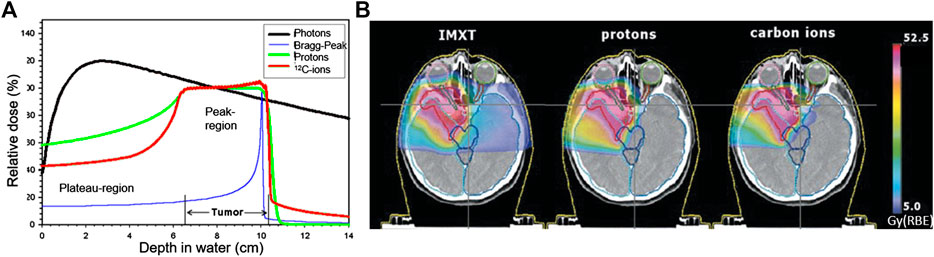
FIGURE 2. Depth dose profiles in water (A) and treatment plans (B) [6] comparing photons, delivered with the most advanced intensity modulated X-ray RT (IMXT), and state-of-the-art scanned protons and C-12 ions, showing the increased tumor-dose conformity of ion therapy due to the characteristic Bragg peak (A).
An important aspect that needs to be addressed is the geographical inhomogeneous distribution of the PT centers in Europe. Figure 3 shows that the majority of Western Europe has access to PT whereas 26 centers8 provide proton therapy for the citizens of Germany (six centers), United Kingdom (5), France (3), Italy (3), The Netherlands (3), Austria (1), Spain (1), Sweden (1), Switzerland (1), Poland (1), and Denmark (1), while South-East Europe with a population of about 40 million inhabitants has not a single PT facility yet. The SEEIIST project is currently in a technical design phase, thanks to the first financial support of the EC (Directorate for Research and Innovation DG RTD). The hosts of this phase are the renowned institutions, such as CERN in Geneva and GSI9 in Darmstadt. The task of the SEEIIST facility is twofold: cancer treatment and associated research program, which should ultimately become an integral part of the PT field.
In order to ensure the future operation of the facility, it is necessary to develop highly qualified trained personnel and the technical capacity in parallel to the design and construction of the SEEIIST facility. This effort will be supported by collaborating with the European Network for Light Ion Hadron Therapy (ENLIGHT)10, established nearly 20 years ago to strengthen EU-PT in clinical research, in R&D for technology and in education and training, based on the principle of open collaboration.
European epidemiological studies preliminary to the Italian, Austrian and French carbon therapy projects made it possible to establish a consensus of priority cases for this type of therapy. From these studies, it was found that the cases eligible for hadron therapy account for about 10% of all radiotherapy patients, which are about 25,000 patients per 10 million inhabitants. About 1% out of this 10% are considered in the very first level of priority. The entire SEE region is covering about 40 million inhabitants and at present there is no medical treatment facility using either proton or heavy-ions in that region yet. Therefore around 1,000 patients will the very first level of priority [6]. As a whole, it can be emphasized that for most of the cases it is a question of rare tumors, the recruitment of which, in order to obtain a particle therapy decision, requires at the first place a healthcare system that is efficient and able to handle all types of cancers and to cover the entire population in an equitable manner. Recruiting them will be one of the main challenges of this initiative. SEEIIST’s projected ability is to treat around 400 patients/year, and after 3 years of activity about one third of the theoretical needs of the Region populations regarding medical cases of highest priority for ion therapy. Those numbers can be gradually increased to 1000 patients over time, mainly by allocated longer beam-time for treatments and/or by upgrading the SEEIIST facility with up to three more treatment rooms.
SEEIIST Therapy Facility Design
The SEEIIST design will significantly move beyond the current state-of-the-art technology used at the operational facilities in the EU. The envisaged technological improvement will allow Europe to compete with Japan, a current leader in Carbon medical facilities, and will further increase its lead over developments in the United States. The following will be the innovative and beyond state-of -the-art aspects in the SEEIIST facility design:
• Outstanding Beam Intensity, higher than the current European centers (HIT [7], CNAO [8], MedAustron [9]) and the present record intensity realized in Japan [10].
• Flexible Dose Delivery system, to deliver the standard slow-extracted beam for active painting of the tumor in a time efficient way, and to achieve dose rates >50 Gy/s, for research purposes and eventually for so called FLASH treatment (irradiation with short impulses and higher intensity beams)
• Flexibility of using different ion species, to support a wide experimental program covering all of the new treatment modalities and providing different ion species from protons to argon, focusing in particular on helium, carbon and oxygen.
• Compact design, Lower Construction and Operation Costs, to achieve a smaller footprint and about 30% lower construction and operation costs for the accelerator with respect to existing facilities, thanks to an extended use of superconductivity and other modern accelerator technologies.
• Effective Beam-time sharing solutions. There will be a highly detailed program developed for the sharing of the beam-time. The treatment rooms (HL, HL + VL, and Superconducting Gantry) are independent of experimental rooms for research (served by two separate beamlines). The patient treatment and animal studies will be space- and function-wize completely independent.
• Green Infrastructure. In addition, SEEIIST will be the first High Energy Physics green infrastructure in Europe. It will be powered by a solar panel photovoltaic farm or a wind farm, hence keeping the facility 100% carbon neutral. A detailed sustainability plan will be drawn up for the SEEIIST RI. It is expected that the patient treatment will ensure full financial sustainability of the facility. The production of isotopes with the injector linac, in particular for PET imaging which can be delivered to hospitals in the region, will also contribute to this financial sustainability. With a specific time-planning and beam management, SEEIIST will dedicate 50% of the beam time for patient treatment and 50% for research and training purposes. The machine will be designed in a manner that can be expanded from the initial configuration. At the exit of the injector linac, a space will be reserved for the medical radioisotope production facility.
SEEIIST Facility – Accelerator Technical Choices
While the proton therapy is well commercialized and production of proton beams is based on compact and relatively cheap machines, the Carbon therapy requires much higher beam energies and therefore larger and more complex accelerator systems.
The production of proton beams for therapy is done by cyclotrons11 or small synchrotrons12 whose footprint is less than 10 m × 10 m. Carbon ions, due to higher stopping power and smaller charge-to-mass ratio, require three times larger synchrotrons or three times stronger magnets. As a positive aspect, accelerators capable of producing therapy carbon ions can also serve for acceleration of protons and other light ion species to the energies required by therapy. All of the 13 currently operational carbon therapy centers in the world are based on synchrotrons [11]. The alternative, on-going developments are cyclotrons [12], linacs [13], rapid-cycling synchrotron [14] and FFA [15].
Cyclotron producing carbon beam is a very large and heavy machine which produces lots of beam losses and has a fixed-energy output, meaning that degraders have to be used for energy reduction. Linacs, currently under development, are expected to provide energy variation at a 100 Hz rate, which is very promising for therapy applications. However, since one of the major features of SEEIIST, as a research laboratory, is the capability to switch to various ion species, linacs are not very flexible in this respect. Also, the linac solution is still in an intensive R&D phase. Rapid-cycling synchrotrons and FFA have also been discarded as alternatives for SEEIIST as the technology is not mature yet and they do not provide significant advantage in terms of cost or reducing footprint and saving space. Therefore, it was concluded that a traditional synchrotron technology should be used for SEEIIST and, as a long-term development option, a synchrotron with super-conducting magnets will be studied to reduce the footprint and complexity and make it comparable to proton synchrotrons.
The main technical elements of the SEEIIST carbon therapy center are depicted in Figure 4: two to four ion sources allowing for a fast change of the accelerated ion species, injector linac, which accelerates the beam to energies of about 4–10 MeV/u, a synchrotron with about 60–80 m of circumference (in case of normal conducting magnets) which brings the beam energy up to the required maximum 430 MeV/u and high energy beam transfer lines which bring the extracted beams to the patients or to the experimentalists and researchers.
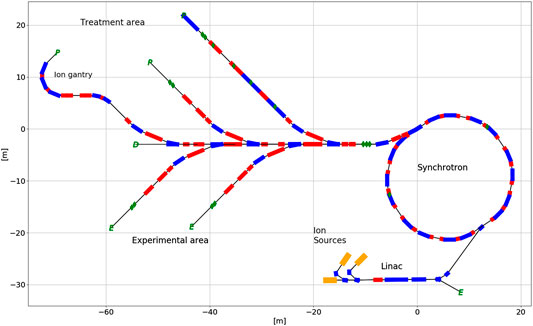
FIGURE 4. A preliminary layout of the SEEIIST facility. The upper beamlines are dedicated to patient treatment while the lower ones are dedicated to radiobiology and materials research.
The main parameters of ion sources critical for carbon therapy applications are: intensity, emittance, and reliability. Currently all centers use Electron Cyclotron Resonance (ECR) ion sources, which provide very stable beams and have very high reliability. The technical developments focus on increase of the ECR source currents and on development of Electron Beam Ion Source (EBIS). EBIS sources offer significantly smaller emittance, that can potentially lead to better transmission in the following injector linac and to significant increase of beam intensity in the synchrotron due to higher efficiency of multi-turn injection. Reliability, though still remains to be proven. The injector linac accelerates the ions from initial energy from the ion source (10–30 keV/u) to injection energy of the synchrotron, which is in the range of 4–10 MeV/u, with optimum around 7 MeV/u. The transmission and final beam energy are the main physical parameters of the linac. It is also a rather expensive system; therefore, the accelerator team is developing a cost-competitive solution for SEEIIST, e.g., by using higher RF frequency which would allow to power up the whole system with a single klystron.
The function of the synchrotron is to accelerate the beams to final energies and extract them in what is called a slow extraction process to the transfer lines and to the patient. The synchrotrons are made of normal conducting magnets which is limiting their circumference to a minimum of about 60 m. The option to use superconducting magnets could cut down the circumference to about 30 m, which is comparable with proton therapy machines.
Several lattice options have been developed for normal-conducting medical synchrotrons. In Europe two distinct approaches have been followed, one proposed by GSI and the other one by CERN/PIMMS [16]. The focus of GSI design was set on compactness and reduced complexity of the machine, while PIMMS was focused on flexibility. As a result of these efforts, the Heidelberg Ion Therapy (HIT)13 synchrotron is about 10 m shorter than the PIMMS one, and the number of components is also significantly reduced. HIT design was taken over by industry, optimized, and two other facilities based on this design were built: in Marburg and in Shanghai, before Siemens withdrawal from ion cancer therapy market. The PIMMS design went through cycles of improvements and its two implementations: CNAO14 and MedAustron, are very mature.
Both European designs, even if the lattices are different, share similar characteristics and in particular they all provide only up to maximum of 109 Carbon ions per cycle, have a similar circumference size, the same linac and source design. Combining the experience of the two major research laboratories behind their designs, our goal is to make SEEIIST accelerator facility compact, flexible and less complex.
Currently, in order to prove feasibility with regard to the major project challenges, it has been assumed that an upgraded design based on PIMMS study [17] and CNAO implementation will serve as a baseline for SEEIIST, with other lattice options explored in parallel. A second long-term development foresees the use of superconducting magnets [18]. Figure 5 shows the SEEIIST (HITRI-design) footprint of the facility if superconducting synchrotron and gantry are used, in comparison to the footprints of the existing ion therapy infrastructures in Europe (CNAO and MedAustron).
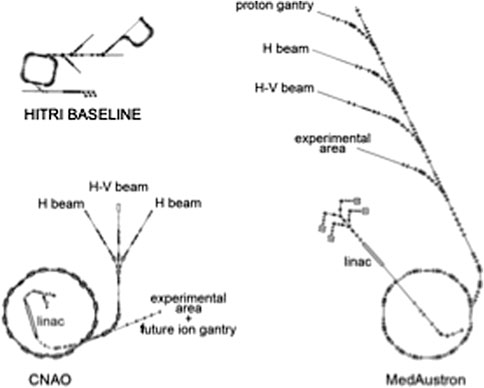
FIGURE 5. Overall size of the SEEIIST (HITRI) footprint compared to CNAO and MedAustron ion treatment facilities (cfr. Figure 4 in [Ref. UA 19).
The main challenge for the SEEIIST (HITRI) design is the increase of the beam intensity. This will be achieved by the increase of ion source intensity, transmission through the linac and optimization of the multi-turn injection process. The slow extraction process from a synchrotron is a complex set of procedures and the quality of the extracted beam depends on machine parameters. Optimization of this process and development of new techniques is a subject of active ongoing research. In SEEIIST, we propose to use RK-KO technique, which is successfully used in HIT and in Japan. Moreover, extraction from multi-energy flat-top is also proposed as a baseline, to reduce the treatment time.
The final parts of the facility are the beam transfer lines to the patient and to the experimental hall. The transfer lines should transport stable beams of various sizes to the patient. This task is complicated by the particular shape of the beam produced by the slow extraction process (bar of charge). Furthermore, there are also two main approaches to transfer line design present in current facilities: minimalistic one, facilitated by HIT and the one with a maximum flexibility - the PIMMS design, where various functions of the beam line are spread among various magnet groups. SEEIIST approach is a compromise between the two approaches, that allows a lot of flexibility and, at the same time, does not take too much space. The state-of-art dose delivery systems relies on 3D beam scanning and the last part of the beam lines contain fast scanning magnets which allow for application of this 3D technique. In the second construction stage, a superconducting compact and innovative gantry will be installed in the facility [18, 19].
Research in Particle Therapy
The number of particle therapy centers is rapidly growing, especially in Europe [20, 21]. In the European landscape, unlike in the United States, these centers are often built with public funds, and it is therefore common that scientific research has a prominent role in the activities of these clinical centers [22]. The biological effects of protons are similar to X-rays [23], and therefore most of the research in proton therapy centers focuses on medical physics [24]. Range uncertainty is typically tackled with different technologies based on range prediction [25, 26] or verification [27, 28]. However, for heavy ion centers research in radiobiology is prominent, because of the different biological properties of densely ionizing radiation compared to X-rays [29]. Research is essential to justify the higher costs of the heavy ion centers compared to conventional radiotherapy (Figure 6).
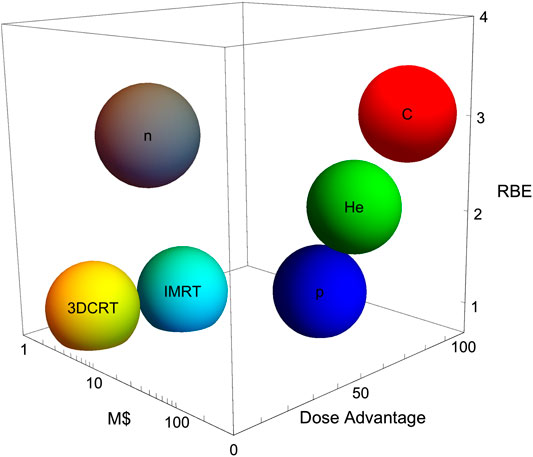
FIGURE 6. The cost-effectiveness of particle therapy. The plot shows physical advantages (dose, in an arbitrary scale), biological advantages (RBE, in a realistic clinic scale), and cost in millions $ for 3D conformal radiotherapy (3DCRT), intensity modulated radiotherapy (IMRT), neutrons and three charged particles (protons, helium, and carbon).
For many years, radiobiological research was focusing on RBE. The topic has been widely and systematically studied in a large number of human and rodent cell lines in Berkeley [30], GSI [31] and NIRS [32]. The results are well known, and summarized in Figure 7 [33]. The RBE increases with LET until reaching a maximum around 100–200 keV/μm, before declining for the overkilling effect. The high variance in Figure 7 reflects the dependence of the RBE, some of them being physical (e.g., the dependence on charge and velocity, rather than LET alone, and on the dose rate) others biological (e.g., the cell-cycle stage or the survival level). One of the main uncertainties is related to the intrinsic radiosensitivity of the cells (or tumor), the so-called 5th R of radiotherapy [34]. However, this is the same uncertainty encountered in clinical practice for establishing the biological effective dose (BED), which is indeed directly dependent on the α/β ratio [35]. Thinking that RBE uncertainty is a showstopper for heavy ion therapy would be similar to state that no fractionation can be done because we do not know the α/β ratio precisely enough.
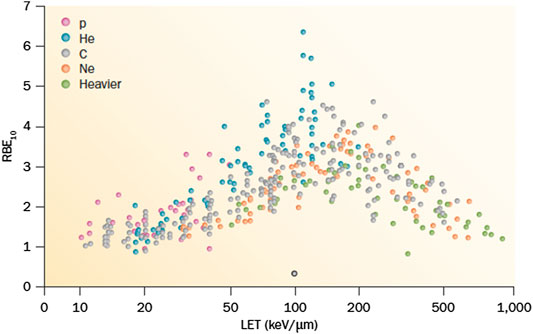
FIGURE 7. A collection of different RBE values for different cell lines as a function of LET. Data from the PIDE database, available online at www.gsi.de/bio-pide.
Being well understood the RBE dependence on LET, modern radiobiological research is shifting toward topics that are also mainstream in conventional radiotherapy, especially with the current emphasis on precision medicine [36]. A few examples are given below, while more comprehensive reviews could be found in Refs. 37 and 38.
Hypoxia
Tumor hypoxia remains one of the worst prognostic factors in cancer therapy [39]. Overcoming hypoxia was one of the main rationales for using heavy ions in the Lawrence Berkeley Laboratory pilot trial in the 70s [40]. Carbon ions, now used in a dozen of centers in Asia and Europe, can only partly solve the problem, because their LET is relatively low and OER goes to one only at LET > 100 keV/μm [41] (Figure 8). While drugs overcoming hypoxia are entering in the clinics [42, 43], strategies based on the physics can certainly contribute in decreasing hypoxia-mediated radio resistance. Kill-painting with carbon ions [44] provides intensity modulation to boost the hypoxic regions, thus overcoming resistance provided that the hypoxic volumes can be visualized by PET before the treatment [45]. Oxygen ions, slightly heavier than carbon, can be more effective against hypoxic tumors maintaining acceptable toxicity [46], and for this very reason they will be used in the Heidelberg Ion Therapy (HIT) clinical center in the coming years for radioresistant cancers [47]. Another approach is to use multi-ions, that can provide high-LET in the target and low-LET in the normal tissue, thus sterilizing the hypoxic tumor with minimal toxicity [48, 49].
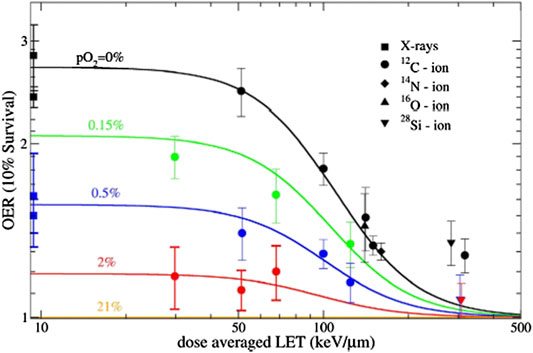
FIGURE 8. Dependence of oxygen effective ratio from LET in CHO cells. Plot from Ref. 44, reproduced under CC BY license from NPG publisher.
Combined Treatments
Immunotherapy of cancer is considered the most promising strategy to reduce mortality, which is largely due to metastatic tumors [50]. However, local treatments remain necessary to tackle the primary tumor and, beyond surgery, radiotherapy has the advantage of eliciting an immune response that can boost immunotherapy [51–53]. Re-activation of immune response is indeed now called the 6th R of radiotherapy [54]. While the recent trials in lung cancer patients have demonstrated the significant survival, advantages expected by combining radio- and immune-therapy [55, 56], the question is whether heavy ion therapy can be more beneficial than X-rays in these combined treatments [57].
This is arguably the most important question for the future of particle therapy, because should radioimmunotherapy by X-rays maintain the promise of largely improving the survival of stage-IV patients, the higher cost of particle therapy (Figure 6) could not be justified. Particle therapy has, however, both physical and biological advantages compared to X-rays in combination with immunotherapy [58]. The main physical advantages are the sparing of the lymphocytes, essential cells to set off an immune response against the metastatic cancer cells. In fact, sparing lymph nodes is now being proposed as a standard practice also for conventional radiotherapy [59]. In addition, cell-death pathways induced by heavy ions seem to be more immunogenic than for X-rays, resulting in enhanced biological effectiveness [60, 61].
SEEIIST and FLASH
Very high-dose radiotherapy (>40 Gy/s) is generally acknowledged as a promising, and potentially evolutionary, pathway for radiotherapy [62, 63]. Pre-clinical data in animal models have indeed shown that at high-dose rate normal tissue toxicity is significantly reduced, while tumor control is not modified [64–66]. The potential advantages in terms of widening the therapeutic windows are enormous. However, reaching these high-dose rates is difficult with X-rays, due to the conversion of electrons in Bremsstrahlung radiation [67]. A first patient has been treated with electrons under FLASH conditions [68], and several proton therapy centers are increasing the cyclotron intensity to reach the FLASH regime [69–71]. For having heavy ion FLASH, high intensity has to be achieved in synchrotrons [72]. This is one of the goals of the new SEEIIST accelerator, as well as of many new accelerators under development worldwide for nuclear research [73]. It is therefore likely that FLASH radiotherapy will be an important topic at SEEIIST, both for clinical and pre-clinical research.
Socioeconomical Benefits of SEEIIST
Before the wars and the crisis in former Yugoslavia, the region had a long history of excellence in science. Before CERN was established in Geneva in 1954, and the International Center for Theoretical Physics in Trieste and the European Molecular Biology Organization in Heidelberg in 1964, former Yugoslavia already had three older research institutes. The Vinča Institute of Nuclear Science in Belgrade was founded in 1948, the Jožef Stefan Institute in Ljubljana in 1949 and the Ruđer Bošković Institute in Zagreb in 1950. Yugoslavia was also one of the founding countries of CERN. However, the scientific progress began to crumble in 1991, along with Yugoslavia’s dissolution and the 1991–2001 wars in former Yugoslavia diminished the economies and science capacity of all countries in the area. An entire generation of young scientists migrated to the Western countries, continuing to do so even in the period after the crisis. This is what gave rise to the political will of the countries of the region and of the EU, “To bring back the tradition in science and technology that the region had in the past.” Furthermore, SEEIIST will revive the scientific and technological potential of the Balkans, whilst helping its economy and bringing people together around a shared endeavor, a vision of a world-leading research institute, built under the same collaborative model as CERN. A €200 million investment in an international research facility in the Balkans could heal the wounds left by the years of ethnic/religious conflicts, help to stop the brain drain and enable the region to regain its former scientific glory. The countries involved in the SEEIIST project hope it will help the region overcome economic difficulties and bring them closer to EU membership.
Inclusion of SEEIIST on the next EU’s roadmap for research infrastructures being drawn up by the European Strategy Forum on Research Infrastructures (ESFRI) in 2021 will be of crucial importance to place the project on the “scientific and political map”.
The average cost of the treatment of a normal patient with heavy ions (not only protons) is estimated to be 25 kEUR in the presently accessible European facilities (HIT, CNAO, MedAustron, and MIT). With the estimated ∼400 patients foreseen to be treated per year in the early phase, SEEIIST will be able to cover 50% of the annual running costs (∼10 MEUR). The remaining cost will be covered by other sources such as membership fees of users (including industry), country contributions (memberships), and research projects from the Horizon Europe programmes.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
A large part of this work has been supported by funding from the EU DG-RTD via a special instrument ‘Service Facility in Support of the Strategic Development of International Cooperation in Research and Innovation N°30-CE-0838742/00-87’.
Conflict of Interest
Author MP was employed by the company COSYLAB.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1https://en.wikipedia.org/wiki/CERN.
3*This designation is without prejudice to positions on status and is in line with UNSC 1244/1999 and the ICJ opinion on the Kosovo Declaration of Independence.
4https://seeiist.eu/start-of-the-seeiist-design-phase-september-2019-budva-montenegro/.
5https://www.who.int/en/news-room/fact-sheets/detail/cancer.
6https://ec.europa.eu/research/.
7“Commission Announces Top Experts to Shape Horizon Europe’s Missions” https://ec.europa.eu/info/news/commission-announces-top-experts-shape-horizon-europe-missions-2019-jul-30_en.
8https://www.ptcog.ch/index.php/facilities-in-operation.
10https://enlight.web.cern.ch/enlight.
11E.g., IBA: https://iba-worldwide.com/proton-therapy/proton-therapy-solutions.
12E.g., HITACHI: https://www.hitachi.com/businesses/healthcare/products-support/pbt/index.html.
13HIT cited before.
14CNAO and MedA cited before.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R-L, Torre L-A, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi:10.3322/caac.21492
2. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groupsA systematic analysis for the global burden of disease study, global burden of disease cancer collaboration. JAMA Oncol (1990) 5(12):1749–68. doi:10.1001/jamaoncol.2019.2996
3. Dagenais G-R, Leong D-P, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet (2020) 395:785–94. doi:10.1016/S0140-6736(19)32092-6
4. Rosenblatt E, Izewska J, Anacak Y, Pynda Y, Scalliet P, Boniol M, et al. Radiotherapy capacity in European countries: an analysis of the directory of radiotherapy centres (DIRAC) database. Lancet Oncol (2013) 14(2):e79–86. doi:10.1016/S1470-2045(12)70556-9
5. Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol (2013) 14(12):1165–74. doi:10.1016/S1470-2045(13)70442-X
6.U Amaldi eds. A facility for tumour therapy and biomedical research in South-eastern Europe. CERN-2019-002. doi:10.23731/CYRM-2019-002
7.NuPECC. F Azaiez, A Bracco, J Dobeš, A Jokinen, G-E Körner, A Majet al. editors Nuclear physics for medicine. Printing: Ireg Strasbourg (2014).
8. Haberer T, Debus J, Eickhoff H, Jäkel O, Schulz-Ertner D, Weber U. The Heidelberg ion therapy center. Radiat Oncol (2004) 73(2):186–90. doi:10.1016/s0167-8140(04)80046-x
10. Benedikt M, Gutleber J, Palm M, Pirkl W, Dorda U, Fabich A. “Overview of the MedAustron design and technology choices,” in Proceedings, 1st international particle accelerator conference. Kyoto, Japan (2010): 186–90.
11. Iwata Y, Kadowaki T, Uchiyama H, Fujimoto T, Takada E, Shirai T, et al. Multiple-energy operation with extended flattops at HIMAC. Nucl Phys Meth A (2010) 624:33–8. doi:10.1016/j.nima.2010.09.016
12. Jongen Y, Abs M, Blondin A, Kleeven W, Zaremba S, Vandeplassche D, et al. “Current status of the iba C400 cyclotron project for hadron therapy,” in Proceedings of IPAC2008. Genova, Italy: CERN (2008) p. TUPP120.
13. Verdú-Andrés S, Amaldi U, Faus-Golfe A. CABOTO, a high-gradient linac for hadrontherapy. J Radiat Res (2013) 54(1):i155–61. doi:10.1093/jrr/rrt053
14. Trbojevic D, Alessi J, Blaskiewicz M, Cullen C, Hahn H, Lowenstein D, et al. “Lattice design of A rapid cycling medical synchrotron for carbon/proton therapy,” in Proceedings of IPAC2011. San Sebastian, Spain: CERN (2011) p. WEPS028.
15. Peach K-J, Aslaninejad M, Barlow R, Beard C-D, Bliss N, Cobb J-H, et al. Conceptual design of a nonscaling fixed field alternating gradient accelerator for protons and carbon ions for charged particle therapy. Phys Rev ST-AB (2013) 16:030101. doi:10.1103/PhysRevSTAB.16.030101
16. Benedikt LM, Bryant P, Crescenti P, Holy P, Knaus P, Maier A, et al. Proton-ion medical machine study (PIMMS)—Part I (1999) p. 232. CERN/PS 99-010 DI Geneva, Switzerland.
17. Bryant PJ, Badano L, Benedikt M, Crescenti M, Holy P, Maier AT, et al. Proton-ion medical machine study (PIMMS) Part 2 (2000) p. 340. CERN-PS-2000-007-DR Switzerland, Geneva.
18. Al Harbi N, Amaldi U, Bergesio D, Garonna A, Ljubicic V, Riboni P, et al. Carbon ion superconducting gantry and synchrotron based on canted cosine-theta magnets. Phys Med Biol (2021).
19. Amaldi U. Oblique raster scanning: an ion dose delivery procedure with variable energy layers. Phys Med Biol (2019) 64:115003. doi:10.1088/1361-6560/ab0920
20. Dosanjh MK, Amaldi U, Mayer R, Poetter R. Enlight. European Network for light ion hadron therapy. Radiother Oncol 128:76–82. doi:10.1016/j.radonc.2018.03.014
21. Grau C, Durante M, Georg D, Langendijk JA, Weber DC. Particle therapy in Europe. Mol Oncol (2020) 14:1492–99. doi:10.1002/1878-0261.12677
22. Durante M. Proton beam therapy in Europe: more centres need more research. Br J Canc (2019) 120:777–8. doi:10.1038/s41416-018-0329-x
23. Tommasino F, Durante M. Proton radiobiology. Cancers (2015) 7:353–81. doi:10.3390/cancers7010353
24. Lomax AJ. Myths and realities of range uncertainty. Br J Radiol (2020) 93:20190582. doi:10.1259/bjr.20190582
25. Bär E, Lalonde A, Royle G, Lu H-M, Bouchard H. The potential of dual-energy CT to reduce proton beam range uncertainties. Med Phys (2017) 44:2332–44. doi:10.1002/mp.12215
26. Johnson RP. Review of medical radiography and tomography with proton beams. Rep Prog Phys (2018) 81:016701. doi:10.1088/1361-6633/aa8b1d
27. Krimmer J, Dauvergne D, Létang JM, Testa É. Prompt-gamma monitoring in hadrontherapy: a review. Nucl Instrum Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip (2018) 878:58–73. doi:10.1016/j.nima.2017.07.063
28. Parodi K. Vision 20/20: positron emission tomography in radiation therapy planning, delivery, and monitoring. Med Phys (2015) 42:7153–68. doi:10.1118/1.4935869
29. Durante M. New challenges in high-energy particle radiobiology. Br J Radiol (2014) 87:20130626. doi:10.1259/bjr.20130626
30. Blakely EA, Ngo F, Curtis S, Tobias CA. Heavy-ion radiobiology: cellular studies. Adv Radiat Biol (1984) 11:295–389. doi:10.1016/B978-0-12-035411-5.50013-7
31. Kraft G. Radiobiological effects of very heavy ions : inactivation, induction of chromosome aberrations and strand breaks. Nucl Sci Appl (1987) 3:1.
32. Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H, et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He−, (12)C− and (20)Ne-ion beams. Radiat Res (2000) 154:485–96. doi:10.1667/0033-7587(2000)154[0485:IOAAHC]2.0.CO;2
33. Friedrich T, Scholz U, Elsässer T, Durante M, Scholz M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J Radiat Res (2013) 54:494–514. doi:10.1093/jrr/rrs114
34. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved?. Int J Radiat Oncol (2014) 88:254–62. doi:10.1016/j.ijrobp.2013.07.022
35. Fowler JF. 21 Years of biologically effective dose. Br J Radiol (2010) 83:554–68. doi:10.1259/bjr/31372149
36. Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Canc (2016) 16:234–49. doi:10.1038/nrc.2016.18
37. Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol (2017) 14:483–95. doi:10.1038/nrclinonc.2017.30
38. Durante M, Flanz J. Charged particle beams to cure cancer: strengths and challenges. Semin Oncol (2019) 46:219–25. doi:10.1053/j.seminoncol.2019.07.007
39. Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Canc (2008) 8:967–75. doi:10.1038/nrc2540
40. Tobias CA. Failla memorial lecture. The future of heavy-ion science in biology and medicine. Radiat Res (1985) 103:1–33. doi:10.2307/3576668
41. Scifoni E, Tinganelli W, Weyrather WK, Durante M, Maier A, Krämer M. Including oxygen enhancement ratio in ion beam treatment planning: model implementation and experimental verification. Phys Med Biol (2013) 58:3871–95. doi:10.1088/0031-9155/58/11/3871
42. Ashton TM, Fokas E, Kunz-Schughart LA, Folkes LK, Anbalagan S, Huether M, et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat Commun (2016) 7:12308. doi:10.1038/ncomms12308
43. McGowan DR, Skwarski M, Bradley KM, Campo L, Fenwick JD, Gleeson FV, et al. Buparlisib with thoracic radiotherapy and its effect on tumour hypoxia: A phase I study in patients with advanced non-small cell lung carcinoma. Eur J Canc (2019) 113:87–95. doi:10.1016/j.ejca.2019.03.015
44. Tinganelli W, Durante M, Hirayama R, Krämer M, Maier A, Kraft-Weyrather W, et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci Rep (2015) 5:17016. doi:10.1038/srep17016
45. Horsman MR, Mortensen LS, Busk M, Overgaard J, Horsman MR, Mortensen LS, et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol (2012) 9:674–87. doi:10.1038/nrclinonc.2012.171
46. Sokol O, Scifoni E, Tinganelli W, Kraft-Weyrather W, Wiedemann J, Maier A, et al. Oxygen beams for therapy: advanced biological treatment planning and experimental verification. Phys Med Biol (2017) 62:7798–813. doi:10.1088/1361-6560/aa88a0
47. Kurz C, Mairani A, Parodi K. First experimental-based characterization of oxygen ion beam depth dose distributions at the Heidelberg Ion-Beam Therapy Center. Phys Med Biol (2012) 57:5017–34. doi:10.1088/0031-9155/57/15/5017
48. Kopp B, Mein S, Dokic I, Harrabi S, Böhlen TT, Haberer T, et al. Development and validation of single field multi-ion particle therapy treatments. Int J Radiat Oncol Biol Phys (2020) 106:194–205. doi:10.1016/j.ijrobp.2019.10.008
49. Sokol O, Krämer M, Hild S, Durante M, Scifoni E. Kill painting of hypoxic tumors with multiple ion beams. Phys Med Biol (2019) 64:045008. doi:10.1088/1361-6560/aafe40
50. Tran E, Robbins PF, Rosenberg SA. “Final common pathway” of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol (2017) 18:255–62. doi:10.1038/ni.3682
51. Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst (2013) 105:256–65. doi:10.1093/jnci/djs629
52. Weichselbaum RR, Liang H, Deng L, Fu Y-X. Radiotherapy and immunotherapy: a beneficial liaison?. Nat Rev Clin Oncol (2017) 14:365–79. doi:10.1038/nrclinonc.2016.211
53. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Canc (2018) 18:313–22. doi:10.1038/nrc.2018.6
54. Boustani G, Grapin M, Laurent P-A, Apetoh L, Mirjolet C. The 6th R of radiobiology: reactivation of anti-tumor immune response. Cancers (2019) 11:860. doi:10.3390/cancers11060860
55. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med (2018) 379:2342–50. doi:10.1056/NEJMoa1809697
56. Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med (2018) 24:1845–51. doi:10.1038/s41591-018-0232-2
57. Durante M, Brenner DJ, Formenti SC. Does heavy ion therapy work through the immune system ? Int J Radiat Oncol Biol Phys (2016) 96:934–6. doi:10.1016/j.ijrobp.2016.08.037
58. Durante M, Formenti S. Harnessing radiation to improve immunotherapy: better with particles?. Br J Radiol (2020) 93:20190224. doi:10.1259/bjr.20190224
59. Lambin P, Lieverse RIY, Eckert F, Marcus D, Oberije C, van der Wiel AMA, et al. Lymphocyte-sparing radiotherapy: the rationale for protecting lymphocyte-rich organs when combining radiotherapy with immunotherapy. Semin Radiat Oncol (2020) 30:187–93. doi:10.1016/j.semradonc.2019.12.003
60. Takahashi Y, Yasui T, Minami K, Tamari K, Hayashi K, Otani K, et al. Carbon ion irradiation enhances the antitumor efficacy of dual immune checkpoint blockade therapy both for local and distant sites in murine osteosarcoma. Oncotarget (2019) 10:633–46. doi:10.18632/oncotarget.26551
61. Ebner DK, Tinganelli W, Helm A, Bisio A, Yamada S, Kamada T, et al. The immunoregulatory potential of particle radiation in cancer therapy. Front Immunol (2017) 8:99. doi:10.3389/fimmu.2017.00099
62. Harrington KJ. Ultrahigh dose-rate radiotherapy: next steps for FLASH-RT. Clin Canc Res (2019) 25:3–5. doi:10.1158/1078-0432.CCR-18-1796
63. Durante M, Brauer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J Radiol (2017) 91:20170628. doi:10.1259/bjr.20170628
64. Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med (2014) 6:245ra93. doi:10.1126/scitranslmed.3008973
65. Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Canc Res (2019) 25:35–42. doi:10.1158/1078-0432.CCR-17-3375
66. Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci USA (2019) 116:10943–51. doi:10.1073/pnas.1901777116
67. Bazalova‐Carter M, Esplen N. On the capabilities of conventional x‐ray tubes to deliver ultra‐high (FLASH) dose rates. Med Phys (2019) 46:5690–5. doi:10.1002/mp.13858
68. Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol (2019) 139:18–22. doi:10.1016/j.radonc.2019.06.019
69. van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel WFAR. Bringing FLASH to the clinic: treatment planning considerations for ultrahigh dose-rate proton beams. Int J Radiat Oncol (2020) 106:621–9. doi:10.1016/j.ijrobp.2019.11.011
70. Diffenderfer ES, Verginadis , Kim MM, Shoniyozov K, Velalopoulou A, Goia D, et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol (2020) 106:440–8. doi:10.1016/j.ijrobp.2019.10.049
71. Patriarca A, Fouillade C, Auger M, Martin F, Pouzoulet F, Nauraye C, et al. Experimental set-up for FLASH proton irradiation of small animals using a clinical system. Int J Radiat Oncol (2018) 102:619–26. doi:10.1016/j.ijrobp.2018.06.403
72. Colangelo NW, Azzam EI. The importance and clinical implications of FLASH ultra-high dose-rate studies for proton and heavy ion radiotherapy. Radiat Res (2019) 193:1–4. doi:10.1667/RR15537.1
Keywords: SEEIIST, Research Infrastructure, Particle Therapy, Cancer Treatment, South East Europe
Citation: Amaldi U, Benedetto E, Damjanovic S, Dosanjh M, Durante M, Georgieva P, Haberer T, Plesko M, Ristova M, Rossi S, Sammut N, Sapinski M, Schopper H, Specht H, Voss R, Vretenar M and Wenninger H (2021) South East European International Institute for Sustainable Technologies (SEEIIST). Front. Phys. 8:567466. doi: 10.3389/fphy.2020.567466
Received: 29 May 2020; Accepted: 26 November 2020;
Published: 29 January 2021.
Edited by:
Wouter van Elmpt, Maastricht University, NetherlandsReviewed by:
Dimitris Emfietzoglou, University of Ioannina, GreeceTom Depuydt, University Hospitals Leuven, Belgium
Copyright © 2021 Amaldi, Benedetto, Damjanovic, Dosanjh, Durante, Georgieva, Haberer, Plesko, Ristova, Rossi, Sammut, Sapinski, Schopper, Specht, Voss, Vretenar and Wenninger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanja Damjanovic, Sanja.Damjanovic@cern.ch
†Deceased
 Ugo Amaldi1
Ugo Amaldi1  Sanja Damjanovic
Sanja Damjanovic Manjit Dosanjh
Manjit Dosanjh Marco Durante
Marco Durante Petya Georgieva
Petya Georgieva Thomas Haberer
Thomas Haberer Mimoza Ristova
Mimoza Ristova Nicholas Sammut
Nicholas Sammut Rudiger Voss
Rudiger Voss