Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1455
Peer-review started: June 16, 2020
First decision: September 18, 2020
Revised: September 28, 2020
Accepted: October 23, 2020
Article in press: October 23, 2020
Published online: December 26, 2020
Metformin is a first-line medication for type II diabetes. Numerous studies have shown that metformin not only has hypoglycemic effects, but also modulates many physiological and pathological processes ranging from aging and cancer to fracture healing. During these different physiological activities and pathological changes, stem cells usually play a core role. Thus, many studies have investigated the effects of metformin on stem cells. Metformin affects cell differentiation and has promising applications in stem cell medicine. It exerts anti-aging effects and can be applied to gerontology and regenerative medicine. The potential anti-cancer stem cell effect of metformin indicates that it can be an adjuvant therapy for cancers. Furthermore, metformin has beneficial effects against many other diseases including cardiovascular and autoimmune diseases. In this review, we summarize the effects of metformin on stem cells and provide an overview of its molecular mechanisms and clinical prospects.
Core Tip: The effect of metformin on stem cells is quickly gaining attention, because metformin modulates various physiological activities and pathological changes via targeting stem cells. Emerging studies suggest that metformin has broad prospects in the fields of stem cell medicine, gerontology, regenerative medicine, and cancer therapy, etc. In this review, we summarize the effects of metformin on stem cells and provide an overview of its molecular mechanisms and clinical prospects.
- Citation: Jiang LL, Liu L. Effect of metformin on stem cells: Molecular mechanism and clinical prospect. World J Stem Cells 2020; 12(12): 1455-1473
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1455.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1455
Metformin (N,N′-dimethyl metformin), which is widely used in patients with type 2 diabetes, exerts hypoglycemic effects mainly by inhibiting absorption of glucose in the gut, suppressing gluconeogenesis and glycogen synthesis, and facilitating the uptake and utilization of glucose, and sensitivity to insulin of peripheral tissues[1]. It is widely accepted that metformin reduces diabetic risk factors such as obesity and improves diabetic complications such as cardiovascular disease, peripheral neuropathy, and higher fracture risk[2-5].
In recent years, studies have shown that metformin modulates many physiological and pathological processes ranging from aging and cancer to fracture healing[1,6-8]. In 2005, Evans et al[9] found that metformin reduces the morbidity of malignant tumors in patients with type 2 diabetes, attracting attention to explore the connection between metformin and cancer[9]. In 2013, Cabreir’s research on the anti-aging effect of metformin was published in the journal Cell. He reported that metformin increases the lifespan of Caenorhabditis elegans cocultured with Escherichia coli by altering microbial folate and methionine metabolism, demonstrating the anti-aging effect and mechanism of metformin[10]. These studies suggest that metformin has regulatory effects on various physiological activities and pathological changes. Studies have shown that stem cells play a curial role in these processes. Therefore, many scientists have studied the effect of metformin on stem cells in recent years.
Previous studies have demonstrated that metformin affects stem cell differentiation, enhances their immunomodulatory properties, and exerts anti-aging, anti-oxidative, and anti-inflammatory effects in stem cells[11-16]. This review focuses on the multiple effects of metformin on stem cells, its molecular mechanisms, and clinical prospects.
Cell differentiation refers to the process through which cells from the same source gradually produce cell groups with different morphological structures and functional characteristics. It is the basis of ontogeny that is conductive to improve the efficiency of various physiological functions. Thus, a large number of studies on stem cell differentiation have been reported. Studies have shown that metformin affects the differentiation of stem cells and progenitor cells[11,17,18]. We have summarized these effects and their molecular mechanisms (Table 1).
| Role of metformin | Stem cell type(s) | Suggested mechanism(s) | Ref. |
| Promoting osteogenic differentiation | BMPC; ADSC; UC-MSC; iPSC-MSC | LBK1/AMPK activation | [20-23] |
| BMSC | AMPK activation-Runx2 (serine 118) | [26] | |
| MC3T3-E1 | AMPK/Gfi1/OPN axis; SIRT-6/NF-κB | [12,24] | |
| hBMSC | Twist1 inhibition; GSK3β/β-catenin/Wnt signaling pathway | [19,25] | |
| PDLSC | AKT/Nrf2 | [27] | |
| ADSC; PDLSC; hDPSC | None | [8,11,32,33] | |
| Promoting neuronal differentiation | NPC | aPKC/CBP | [35] |
| hBMSC | AMPK activation | [36] | |
| NPC | AMPK/aPKC/CBP signaling pathway | [37] | |
| hiPSC-NSC | None | [17] | |
| Promoting myogenic differentiation | Satellite cell | RPS6-mTOR | [14] |
| C2C12 | ERK; AMPK (AMPKα1)/HDAC5 | [40,41] | |
| Muscle progenitor cell | AMPK | [39] | |
| Inhibiting adipogenic differentiation | MC3T3-E1 | AMPK/Gfi1/OPN axis | [24] |
| MSC | AMPK/mTOR/p70S6K | [44] | |
| ADSC; PDLSC; BMSC; BMPC | None | [13,27,42,43,46] | |
| Inhibiting chondrogenic differentiation | ATDC-5 | AMPK | [47] |
| Gastric PC differentiation | Gastric EPC | AMPK | [50] |
| Regulating stem cell aging and rejuvenating regeneration | HMSC | Nrf2/GPx7 | [59] |
| ISC | AKT/TOR/Atg6-related pathway; AKT/mTOR pathway | [62-64] | |
| Satellite cell | mTOR /p70S6 | [14] | |
| OPC | AMPK activation | [68] | |
| Inhibiting CSCs | CSC | Hedgehog, Wnt, and TGF-β pathways | [72] |
| Glioblastoma CSC | C1CL1 | [74] | |
| Colorectal cancer CSC | MIF/CD74 axis | [77] | |
| Breast CSC | MiR708/CD47 axis | [81] | |
| CSC | None | [73,76,82] | |
| Improving EPC functions and angiogenesis | EPC | AMPK/eNOS/NO signaling pathway; AMPK/mTOR/autophagy pathway; AMPK/mTOR/p70S6K pathway | [89,90] |
| Antioxidant | ADSC, C2C12 | ROS&NO reduction/SOD activation | [13,40,99] |
| PDLSC | AKT-Nrf2 signaling pathway | [27] | |
| HMSC | Nrf2/GPX7 | [59] | |
| hNSC | AMPK activation | [98,100,102] | |
| Anti-inflammatory | hNSC | AMPK/(IKK/NF-κB) | [15] |
| rabbit AFSC | HMGB1 | [104] | |
| Immunomodulatory potential | ADSCs | AMPK/mTOR/STAT-1 signaling pathway | [107] |
Bone is a complex tissue containing several cell types, which is continuously undergoing a process of self-renewal and repair, termed bone remodeling. Many studies have indicated that metformin promotes osteogenic differentiation of stem cells and osteogenic progenitor cells. The promotive effects manifest as increased cell proliferation, cell migration, alkaline phosphatase activity, mineral deposition, and upregulated expression of osteoblast marker genes, including osteopontin (OPN), osteocalcin, and runt-related transcription factor 2 (Runx2), during osteogenic cell differentiation[8,11,19].
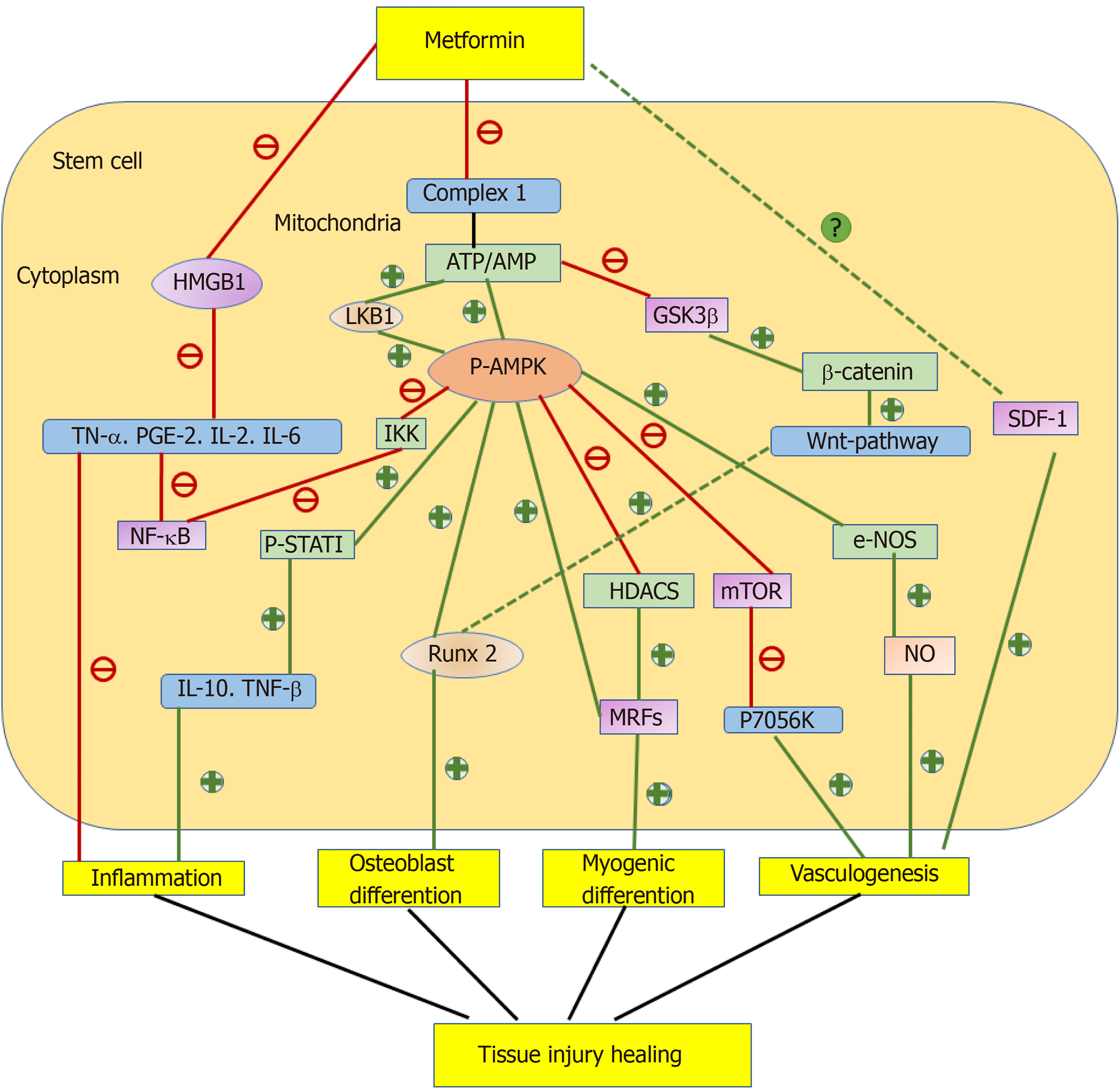
Metformin promotes osteogenic differentiation mainly through the adenosine 5′-monophosphate-activated protein kinase (AMPK) signaling and Runx2-related signaling pathways[20-27]. Metformin is an AMPK activator similar to 5-aminoimidazole-4-carboxamide ribonucleotide[28]. Its primary site of action is direct inhibition of complex 1 of the respiratory chain, which decreases production of ATP, leading to an increase of the AMP/ATP ratio and then activated AMPK[29]. Sedlinsky et al[21] submitted bone marrow progenitor cells (BMPCs) to 15 d osteoblastic induction in the presence or absence of metformin and/or compound C (an inhibitor of AMPK activation). As a result, metformin increased the P-AMPK/total AMPK ratio and production of type 1 collagen (a marker of osteoblastic differentiation) in BMPCs, whereas compound C inhibited these increases, demonstrating that metformin promoted osteoblastic differentiation of BMPCs through AMPK activation[21]. Similarly, Wang et al[23] treated induced pluripotent stem cell (iPSCs) with metformin, demonstrating the same effect via the liver kinase B1 (LKB1)/AMPK signaling pathway. LKB1 is a common upstream molecule of AMPK kinase. Inhibiting its activity markedly reverses metformin-induced AMPK activation and Runx2 expression[23]. In addition, metformin exerts a similar effect on MC3T3-E1 cells through the AMPK/growth factor independence-1 (Gfi1)/OPN axis. AMPK activation downregulates the transcriptional repressor Gfi1 and disassociates it from the OPN promoter, ultimately upregulating OPN[24]. Furthermore, metformin may promote osteoblastic differentiation through decreased acetyl coenzyme carboxylase activity and lipogenic enzyme expression induced by AMPK activation. These decreases contribute to inhibited adipogenesis and break the balance between osteogenic and adipogenic differentiation[30].
Regulation of the Runx2-related signaling pathway by metformin is the second mechanism to promote osteogenic differentiation. Runx2 promotes mesenchymal stem cells (MSCs) to differentiate into preosteoblasts and inhibits adipogenic and chondrogenic differentiation[31]. Marofi et al[25] treated human bone marrow stromal cells (hBMSCs) with metformin and found that metformin promoted osteogenic differentiation through the Twist1/Runx2 signaling pathway. Metformin inhibited the expression of Twist1 by enhancing its gene promoter methylation slightly and a direct physical interaction without Twist1 methylation. Lower Twist1 expression increased the mRNA expression of Runx2[25]. In addition, Chava et al[26] extracted BMSCs from metformin-treated rats and demonstrated that metformin promoted osteogenic differentiation through AMPK directly mediating Runx2 phosphorylation at serine 118[26].
In addition to the two abovementioned signaling pathways, metformin promotes osteogenic differentiation through other mechanisms. Metformin promotes osteogenic differentiation through the serine/threonine kinase Akt (also known as protein kinase B or PKB) signaling pathway. Jia et al[27] treated periodontal ligament stem cells (PDLSCs) with metformin and found that metformin rescued osteogenic differentiation of PDLSCs, which was impaired by H2O2-induced oxidative stress by activating Akt and downstream nuclear factor E2-related factor 2 (Nrf2), an important transcription factor against oxidative stress[27]. Ma et al[19] treated hBMSCs with metformin and obtained the similar result. They stated that the Wnt/â-catenin signaling pathway probably participated in the osteogenic differentiation of BMSCs because metformin inhibited glycogen synthase kinase-3â, resulting in accumulation of cytosolic â-catenin and activation of the Wnt signaling pathway[19].
The effect of metformin on osteogenic differentiation may be influenced by the drug dose, cell origin, and glucose concentration. Several studies explored the effect of metformin on osteogenic differentiation of PDLSCs and found that 50 ìmol/L was the optimal concentration to exert effects[11,27,32]. Houshmand and Ma studied the same effect on BMSCs and hDPSCs, and found that 100 ìmol/L was the optimal concentration to promote cell osteogenic differentiation[19,33]. Moreover, Mu et al[12] found that metformin promoted osteogenic differentiation of murine preosteoblasts under high glucose conditions. In this study, metformin suppressed the phosphorylation of nuclear factor-kB by increasing silent information regulator (SIRT)-6 expression. High levels of SIRT6 will decrease mature osteoblast functions and delay maturation of bone matrix [12,34].
The nervous system has a complex structure and is the major controlling, regulatory, and communicating system in the body. However, unfortunately, when brain cells are damaged by trauma or disease, they are unable to automatically regenerate, which determines nervous dysfunctions and onset/progression of neurodegenerative diseases. Neurodegenerative diseases/neurodegenerative pathologies, including Huntington’s disease, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, represent a group of illnesses characterized by the following features: A decline in neuronal functions, brain atrophy, and often, abnormal deposition of proteins. Several studies have shown that metformin promotes the proliferation and neural differentiation of stem cells[17,35-37], indicating that metformin may be a promising drug for prevention and treatment of these diseases[38].
Ahn et al[36] treated hBMSCs with metformin and demonstrated that metformin may promote neuronal differentiation and neurite outgrowth by activating AMPK. Neuronal cells were characterized by an increase in the expression of neuron-specific genes MAP-2, Tuj-1, NF-M, KCNH1, and KCNH5[36]. Dadwal et al[35] extracted subependymal-derived neural precursor cells (NPCs) and plated them as single cells to form neurospheres in the presence of metformin. The results showed that metformin expanded the stem cell pools and facilitated neurogenesis in normal mice compared with CREB-binding protein (CBP) gene mutant mice, demonstrating that metformin directly promoted NSCs to differentiate via the atypical protein kinase C (aPKC)-CBP pathway[35]. Fatt et al[37] added metformin to NPCs extracted from the adult subventricular zone and found that metformin significantly enhanced neuronal differentiation by activating the AMPK-aPKC-CBP pathway[37].
Skeletal muscle is the largest organ of the body and plays an important role in essential life activities such as respiration, metabolism, mediating temperature, and movement. When empyrosis, trauma, and other factors cause damage to skeletal muscle, skeletal muscle can be regenerated. Skeletal muscle regeneration is dependent on a contribution from muscle-resident stem cells, named satellite cells marked by paired-box transcription factor 7 (Pax7)[39]. The effects of metformin on satellite cells are disputed. Several studies provide evidence that metformin maintains satellite cells in a low differentiation state and deplete skeletal muscle regeneration via calorie restriction, whereas others have stated that metformin alleviates muscle wasting post-injury[14,18,39].
A family of myogenic regulatory factors (MRFs), such as myogenic differentiation antigen (MyoD), myogenin, Mrf4, and myogenic factor (Myf5), plays an important role in myogenic differentiation[18,31]. Pavlidou et al[18] found that metformin (2-10 mmoL/L)-treated C2C12 cells had a reduced myogenic differentiation potential and significant decline in the expression of myogenic regulatory factors MyoD, myogenin, and myosin heavy chain[18]. In another study, they treated satellite cells with 2 mmoL/L metformin in vitro, resulting in retained expression of Pax7 for a longer time, whose delayed downregulation was accompanied by late expression of myogenic differentiation markers, indicating delayed differentiation. In vivo, metformin delayed regeneration of cardiotoxin-damaged skeletal muscle[14]. Conversely, Yousuf et al[39] injected metformin hydrochloride dissolved in phosphate buffered saline into mice after burn injury and found that metformin enhanced the proliferative activity of Pax7-positive satellite cells by activating AMPK. They attributed the contradictory conclusion between the results to different mouse mobility and the different nature of the injury[39].
The effect of metformin on myogenic differentiation of C2C12 cells is controversial and may be related to drug dose. Low doses of metformin (400 and 500 ìmol/L) promote myogenic differentiation and myotube formation, increasing the protein expression of Myf5 and MyoD, two important markers of early differentiation. Senesi et al[40] believed that metformin enhanced C2C12 differentiation by activating ERKs and decreasing p70S6 kinase[18,40]. Fu et al[41] inferred that metformin facilitated myogenic differentiation of C2C12s by activating AMPK. AMPKá1 phosphorylated histone deacetylase 5 (HDAC5) at Ser 259 and 498 in C2C12 cells, which acts as a conserved transcriptional repressor through an interaction with myocyte enhancer factor-2. Phosphorylated HDAC5 upregulates myogenin transcription and myogenesis[41]. Considering the paradoxical effect of metformin on myogenic differentiation, more studies in this field are needed.
Considering the reciprocal relationship between osteogenic and adipogenic differentiation, various reasons, such as diabetic conditions and the use of thiazolidinedione, cause active adipogenesis in BMSCs/BMPCs, which consequently suppresses osteogenesis and damages bone health[42-44]. Metformin inhibits adipocyte differentiation of adipose-derived stem cells (ADSCs), BMSCs, BMPCs, and PDLSCs, manifesting as suppressed cell proliferation, lipid droplet generation, and expression of adipocyte genes such as peroxi-some proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer binding protein alpha, and adipocyte lipid-binding protein[13,27,42,43,45,46].
Marycz et al[13] extracted ADSCs from metformin-treated rats to induce adipogenic differentiation. A reduction of lipid droplets in ADSCs and decreased proliferation potential demonstrated that metformin inhibited adipogenic differentiation[13]. Tolosa et al[42] extracted BMPCs from diabetic rats, treated them with/without metformin, and found that metformin partially abolished diabetic-related upregulation of PPARã expression[42]. Similarly, Molinuevo et al[46] treated BMPCs with both metformin and rosiglitazone and found that metformin abolished rosiglitazone-induced adipogenesis[46].
Metformin inhibits adipogenic differentiation through the AMPK signaling pathway. Wang et al[24] established LV-AMPKá 3T3-L1 cells stably overexpressing AMPKá through a lentiviral vector and treated them with puromycin to induce their adipogenic differentiation. The results showed that activated AMPK suppressed lipid droplet generation and adipocyte gene expression[24]. The other mechanism was inhibiting the mammalian target of rapamycin (mTOR)/ribosomal protein S6 kinase signaling pathway. Chen et al[44] found that metformin suppressed adipogenesis in C3H10T1/2 MSCs independently of the AMPK signaling pathway by measuring phosphorylation of a known AMPK substrate, Ser 79 of ACC. Lipid accumulation associated with adipogenesis in C3H10T1/2 cells was inhibited by incubation with the mTOR/p70S6 kinase inhibitor rapamycin[44]. In addition, because of the reciprocal relationship between osteoblast and adipocyte differentiation, metformin may inhibit adipocyte differentiation indirectly by promoting the expression of osteoblastic transcription factors[43].
Downregulation of chondrocytic differentiation has been described in various chronic skeletal diseases including osteoarthritis. Metformin appears to inhibit chondroblastic differentiation. Bandow et al[47] treated chondrogenic progenitor cells with metformin during chondrogenic differentiation. As a result, metformin inhibited chondroblastic differentiation by activating AMPK. In primary chondrocyte precursors, metformin decreased gene expression of sex determining region Y-box (Sox) 9 and Sox6 along with other chondrogenic differentiation markers including collagen, type II, alpha 1 (col2a1), and aggrecan core protein (ACP). Col2a1 and ACP promoter activities were directly repressed by AMPK-activated early growth response-1 (Egr-1), a transcriptional repressor in mouse chondrocytes independent of Sox9. Mutation of the putative Egr-1-binding site abrogated the inhibitory effects of an AMPK activator[47]. Sox9 plays an important role in various stages of chondrogenesis and is essential for chondrogenesis. Its gene deletion can lead to achondroplasia[48].
Metformin has been reported to reduce the risk of stomach cancer by up to 51% in diabetic patients following eradication of Helicobacter pylori[49]. A recent study showed that metformin promotes differentiation of gastric epithelial progenitor cells into acid-secreting PCs through AMPK activation. AMPK activation increased Kruppel-like factor 4 (KLF4) expression, facilitating progenitor cells to exit the cell cycle and differentiate toward the PC lineage. AMPK appeared to increase maturation of the PC lineage largely by peroxisome proliferator-activated receptor-ã coactivator-1á activation[50]. Considering that PC damage plays a crucial role in the occurrence and development of gastric cancer, metformin may have potential as an anti-gastric cancer drug by promoting gastric epithelial progenitor cells to differentiate into acid-secreting PCs.
Studies showed that metformin and stem cells also play an important role in tissue injury healing, which may relate to the effect of metformin on stem cell differentiation[51-53]. Stem cells have been demonstrated to promote tissue injury healing such as diabetic foot ulcer, burns, gastric ulcer, and ulcerative colitis (UC). They exert the repair effect through migrating to tissue injuries, differentiating into specific cells, reducing inflammation, and producing paracrine factors to promote angiogenesis[51,52]. Deng et al[53] found that bencher muscular dystrophy-endothelial progenitor cells (BMD-EPC) contributed to tissue repair in UC. Notably, stromal cell-derived factor-1 and its receptor CXCR4 also have been demonstrated to play an important role in the “homing” of BMD-EPC to injured sites and neovascularization in tissue repair[53]. While metformin exerts the repair effect through facilitating the process described above, metformin also promotes injury healing through insulin sensitization in diabetic foot ulcer[51,52]. The combination of stem cells and metformin appears to be a better synergistic option for the treatment of diabetic wounds (Figure 1).
Aging can be considered as a developmental program that is beneficial early in life but not switched off upon its completion. From a stem cell-centered perspective, aging results in an impaired regenerative capacity to effectively maintain tissues and organs as well as depletion of stem cell pools in adult tissue, leading to tissue dysfunction and age-associated diseases. For example, the number and proliferation or differentiation ability of stem cells gradually decrease with age. Therefore, damaged tissues and organs cannot be repaired and regenerate in time, which directly leads to the occurrence of human aging and diseases[54]. Both extrinsic (local microenvironment and systemic circulation) and intrinsic factors (genomic instability, DNA damage, oxidative damage, and deteriorated mitochondrial functions) contribute to stem cell dysfunction during aging-related regenerative decline[55-57]. Anti-aging has been a research hotspot in recent years. In 2019, Fahy’s research on reversing “biological age” became the headline of Nature. He found that systemic treatment with a cocktail of growth hormone, dehydroepiandrosterone (DHEA), and metformin partially reverses DNA methylation age (DNAma) clocks. DNAma is a prominent biomarker of mammalian aging[58,59]. It was the first time that clinical research indicated the anti-aging effect of metformin. Thus, metformin is currently undergoing repurposing as an anti-aging agent[6,58,60,61].
Metformin rescues stem cells from aging by alleviating intrinsic undesirable changes. The anti-aging effect of metformin is closely related to its antioxidant effects in stem cells. Fang et al[62] demonstrated that chronic low-dose metformin treatment increases the lifespan of HMSCs through Nrf2-mediated transcriptional upregulation of endoplasmic reticulum-localized glutathione peroxidase 7 (GPx7) whose depletion results in premature cellular senescence[62]. Metformin also inhibits mTORC1 activity, initiating the process of autophagy[55,63]. Autophagy of stem cells maintains internal homeostasis in response to stress conditions and plays a crucial role in stem cell rejuvenation[64]. Na et al[65] found that metformin inhibited aging-related phenotypes in Drosophila midgut intestinal stem cells (ISCs) through the Atg6 (autophagy related gene 6; Beclin 1 in mammals)-related pathway, which was negatively regulated by the AKT/TOR pathway[65]. Metformin reduced age and oxidative stress-related accumulation of DNA damage marked by drosophila ãH2Ax foci and 8-oxo-dG by suppressing the AKT/mTOR pathway in Drosophila midgut ISCs[66,67]. Therefore, inhibiting the AKT/mTOR pathway may decrease DNA damage through activation of autophagy.
Furthermore, metformin is involved in metabolic-induced rejuvenation by mimicking the metabolic effects of calorie restriction. The mechanism may be related to stimulating AMPK, the principal energy sensor in cells, to reduce energy-consuming processes and increase insulin sensitivity[60,64,68,69]. It is commonly accepted that the quiescent state of stem cells, which has been linked to their metabolic state, is correlated with their regenerative capacity[14,57,70]. Pavlido’s research showed that metformin inhibited mTOR activity and reduced p70S6 phosphorylation that induced a low metabolic state associated with quiescence of satellite cells[14]. Similarly, Neumann et al[71] demonstrated that metformin rejuvenated aging oligodendrocyte progenitor cells by activating AMPK[71].
Activation of AMPK by metformin provides an efficient barrier to reprogramming somatic cells (mouse or human fibroblasts) to stem cells[72]. Cellular reprogramming is inducing somatic cells into a pluripotent cell state by expression of transcription factors octamer-binding transcription factor-4 (Oct4), Sox2, Klf4, and c-Myc[73]. Reprogramming reverses many aspects of aging by resetting metabolic signatures, mitochondrial networks, and other factors to a youthful state[69]. Metformin inhibits the reprogramming process by selectively impairing expression of Oct4, but not other reprogramming factors[72,74].
Cancer stem cells (CSCs) are cancer cells with the capacity to renew indefinitely, resulting in tumor formation. They possess stem cell properties including self-renewal, proliferation, and differentiation potential. CSCs are responsible for the clinical failure of the majority of currently available oncological therapies because they survive treatment with hormones, radiation, chemotherapy, and molecularly targeted drugs[75]. Therefore, how to eliminate CSCs has become a research focus in recent years. Many studies have explored the effect of metformin on CSCs and the results are inspiring. Metformin is expected to become an anti-cancer agent[7,9]. The studies showed that metformin inhibits CSCs via inhibition of self-renewal, metastatic, metabolic, and chemoresistance pathways.
Metformin inhibits pathways associated with self-renewal and metastasis in various CSCs. Saini et al[75] stated that the inhibition mechanism included hedgehog, Wnt, and transforming growth factor-â pathways. CSCs self-renew indefinitely, results in tumor formation, and has a potential role in tumor metastasis. Courtois et al[76] confirmed the anti-proliferative effect of metformin on gastric carcinoma cell lines by regulating the expression of genes implicated in cell-cycle regulation (GADD45, p21, E2F1, and PCNA)[76]. Zhao et al[77] demonstrated that metformin inhibits the proliferation of osteosarcoma stem cells (OSCs) by inducing G0/G1 phase cell cycle arrest[77]. In a recent study, Barbieri et al[78] stated that metformin and other biguanides exert anti-proliferative effects in glioblastoma CSCs by interfering with the activity of the extracellular portion of the active transmembrane chloride ion channel. Chloride intracellular channel 1 (CLIC1) activity promotes cell cycle progression and cell division during G1/S phase transition, leading to accelerated growth in glioblastoma CSCs[78].
Metformin also inhibits pathways associated with CSC metabolism. Metabolic reprogramming refers to changes in the metabolic patterns of CSCs compared with normal cells, which makes the body provide sufficient resources for CSCs. A classic example of metabolic reprogramming is the Warburg effect. CSC-driven tumorigenesis through metabolic reprogramming is closely associated with the acquisition of stem cell-like properties in iPSCs. Several studies have demonstrated that metformin suppresses the expression of reprogramming factor Oct4, providing a barrier against malignant CSCs[69,72,79]. Saini et al[75] stated that the metabolic effect of metformin on CSCs mainly depends on inhibition of mitochondrial complex I[75]. It is generally believed that the therapeutic effect of metformin results from an inability of CSCs to turn on glycolysis for ATP production (Warburg effect) upon inhibition of oxidative phosphorylation[80]. A recent study highlighted the strong dependence on energy-producing pathways of colorectal cancer CSCs, suggesting that modulation of AMPK activity is an effective therapeutic approach[81]. Zhao et al[77] showed that metformin induced caspase-mediated apoptosis in OSCs by inducing mitochondrial dysfunction. In addition, metformin influenced the capacity of OSCs to self-renew via enhanced autophagy, which was suppressed by 3-methyladenine, an inhibitor of autophagy[77].
Metformin inhibits CSCs by impairing the chemoresistance of CSCs[82-84]. Tan et al[85] demonstrated that metformin regulates the miR708/cluster of differentiation 47 (CD47) axis to eradicate breast cancer stem cells and enhance chemosensitivity because of the critical role that CD47 plays in evasion of immunological eradication[85]. Bishnu et al[86] showed that continuous metformin treatment impeded acquirement of chemoresistance by reducing the CSC proportion through taurine generation and removing CSCs from quiescence. Maintaining a more proliferative cellular state also contributes to chemosensitivity[86].
The cardiovascular system is also called the circulatory system, consisting of the heart and blood vessels. It transports oxygen and metabolizes waste to maintain steady metabolism of the internal environment and normal life activities. Cardiovascular diseases are associated with impaired vascular remodeling and a lack of endothelial cell reconstructive functions[87]. EPCs, as a kind of precursor cell, have the functions of migration, proliferation, adhesion, and differentiation into endothelial cells involved in the generation of adult neovascularization[88]. Functionally impaired EPCs manifest as decreased EPC numbers in circulation, decreased angiogenic potential, and endothelial dysfunction[89-91]. To our knowledge, most studies have reported that metformin protects the cardiovascular system by improving EPC functions and angiogenesis[92,93].
Metformin improves EPC functions through the AMPK-endothelial nitric oxide synthase (eNOS)-nitric oxide (NO) signaling pathway. Li et al[93] treated EPCs from normal individuals with metformin and found that metformin promoted EPCs to differentiate into ECs. Yu et al[94] treated EPCs from diabetic rats and found that metformin increased EPC capacities for immigration and tube formation. In the two studies, metformin increased both phosphorylated AMPK and eNOS expression in EPCs and enhanced NO production[93,94]. The reduction in NO bioavailability due to reduced synthesis from eNOS can cause EPC dysfunction[95]. Li et al[93] also found that metformin improved EPC functions through AMPK-mTOR-autophagy-related and AMPK-mTOR-p70S6K pathways. The drug activated AMPK and inhibited mTOR[93]. Metformin improved palmitic acid (PA)-induced EPC dysfunction by mediating microRNA (miR) 130a and phosphatase and tensin homolog (PTEN), which may be associated with activation of the phosphatidylinositol-3-kinase/AKT signaling pathway. Levels of miR-130a are lower and those of PTEN are higher in EPCs of diabetic patients[87].
Some studies have suggested negative effects of metformin on EPCs. Metformin attenuates EPC migration through the AMPK/mTOR/autophagy-related pathway. Metformin also activates AMPK phosphorylation and inhibits mTOR and Akt phosphorylation, decreasing matrix metalloproteinase (MMP)-2 and MMP-9 expression in EPCs, indicating that decreased activity of gelatinase and fibrinolysis may contribute to this phenomenon. However, how AMPK/mTOR-related autophagy regulates cell migration is controversial[93,96,97]. The inhibitory effect of metformin on EPC angiogenesis is mediated by down-regulating miR-221 expression, which is negatively correlated with the concentration of metformin and consequently increased p27 expression and activated autophagy[97]. Asadian et al[98] found that metformin inhibited EPC proliferation and angiogenesis following inhibited activation of the Tunica internal endothelial cell (Tie2)/AKT signaling pathway, which may be associated with the Tie2/Akt/eNOS signaling pathway[98,99]. Similarly, Montazersaheb et al[100] found that prolonged incubation with metformin decreased the angiogenic potential of hBMSCs by modulating the mTOR-related autophagy signaling pathway[100]. Considering the paradoxical effects of metformin on EPCs and angiogenesis, one explanation would be that metformin behaves differently according to diabetic conditions and drug concentration[92,100]. Hence, the role of metformin is debatable in EPC functions and needs to be validated by future studies.
Various diseases, such as diabetes, Alzheimer’s disease, and cardiovascular disease, are associated with oxidative stress, and metformin acts as an antioxidant at the cellular level via the mechanisms described below[38,54,101,102]. First, metformin decreases free radicals, including reactive oxygen species (ROS) and NO, and upregulates activities of antioxidant enzymes in stem cells, such as superoxide dismutase (SOD) and ER-located GPx7[13,27,40,62,103,104]. It significantly attenuates ROS production of BM-derived hematopoietic stem cells after total body ionizing radiation irradiation[105]. Low-dose metformin increases the nuclear accumulation of Nrf2 that binds to antioxidant response elements in the GPX7 gene promoter to induce its expression[62]. Advanced glycation endproducts (AGEs) elevate during certain physiological and pathological states including inflammation, aging, diabetes, and neurodegenerative diseases. Human neural stem cells (hNSCs) treated with AGEs have decreased cell growth, but metformin rescues hNSCs from AGE-induced oxidative stress, normalizes ROS levels, and improves SOD activity by decreasing the levels of receptor for advanced glycation endproducts that is downstream of AMPK[104]. Furthermore, metformin protects mitochondria from oxidative damage. Cytosolic cytochrome c activates the caspase protein family, thereby initiating mitochondrion-mediated apoptosis.
Chiang et al[102]’s work focused on hNSCs exposed to amyloid-Aâ or AGEs, which had reduced expression of mitochondrion-associated gene, mitochondrial deficiency (lower displacement loop level, mitochondrial mass, maximal respiratory function, cyclooxygenase activity, and mitochondrial membrane potential), as well as increased activation of caspase 3/9 activity and cytosolic cytochrome c in common. Metformin abrogates these negative effects through the AMPK signaling pathway[102,106].
The anti-inflammatory effects of metformin in neurodegenerative diseases, EPC dysfunction, and aging have also attracted attention in recent years[38,54,107]. Chung et al[106] demonstrated that metformin suppressed AGE-induced inflammation in hNSCs by activation of AMPK, which inhibited inhibitory nuclear factor kappa-B (NF-kB) kinase (IKK) activity and normalized expression of inflammatory cytokines including interleukin (IL)-1á, IL-1â, IL-2, IL-6, IL-12, and tumor necrosis factor á (TNF-á). Decreased NF-êB levels caused by inhibited IKK activity alleviated the inflammatory response via increased expression of inducible nitric oxide synthase (iNOS) and COX-2[15]. Han et al[107] showed that metformin decreases the expression levels of proinflammatory cytokines (IL-1b, IL-6, and TNF-á) by preventing high mobility group box 1 (HMGB1) release from the nucleus to cytoplasm in rabbit annulus fibrosus stem cells. HMGB1 has been proved to play a role in the development and maintenance of the inflammatory response[108]. Furthermore, the senescent phenotype induced by lipopolysaccharide is inhibited by metformin, indicating a correlation between its anti-inflammatory and anti-aging effects[107]. Considering the close relationship between oxidative stress and chronic inflammation, some researchers have suggested that the antioxidant, anti-inflammatory, and anti-aging effects of metformin are interactive[54,101].
Metformin has also shown potential to treat autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, by regulating metabolism[109,110]. A recent study showed that metformin enhanced the immunomodulatory properties of ADSCs through the AMPK/mTOR/signal transducer and activator of transcription (STAT)-1 signaling pathway. Metformin increased AMPK and STAT1 phosphorylation in a dose-dependent manner, but decreased the phosphorylation of mTOR[111]. STAT1 plays an important role in cell survival and proliferation, whose overexpression strongly enhances cord blood-derived MSC-mediated T-cell suppression[112,113]. STAT1 inhibition of ADSCs by metformin significantly impairs induction of immunomodulatory markers, including indoleamine 2,3-dioxygenase, IL-10, and transforming growth factor-â, and inhibits T-cell proliferation in vitro[111].
Various studies show that metformin acts in a dose- and time-dependent manner[114-116]. The adverse effects of metformin on stem cells include decreasing proliferation activity, cytotoxic effects (morphology and ultrastructure changes), and apoptosis when the drug concentration is far more than the therapeutic doses administered to diabetic patients whose plasma concentrations of metformin are usually < 50 ìM. The levels of metformin that accumulate in tissues might be several times higher than that in blood[117]. Œmieszek et al[116] exposed ADSCs and BMSCs to 1, 5, and 10 mmoL/L metformin and then measured cell proliferation activity and characteristic features after 24, 48, and 72 h. They found that metformin inhibited cell proliferation in a dose- and time-dependent manner, and that 5 and 10 mmoL/L metformin had cytotoxic effects on ADSCs, causing abnormal morphological, ultrastructural, and apoptotic changes. The decrease in cell proliferation was associated with cytotoxic effects of metformin[115,116]. Consistent with this result, Jia et al[27] observed that a high concentration of metformin (2500 µM) slightly inhibited cell proliferation of PDLSCs[27]. They suggested that metformin induced hUC-MSC apoptosis in an AMPK-mTOR-S6k1-Bad (Bcl-2 family member)-dependent manner, which was reversed by compound C. Metformin greatly increased the rate of hUC-MSC apoptosis in a dose- and time-dependent manner without affecting autophagy or proliferation[118]. Compared with BMSCs and ADSCs, lower concentrations of metformin (100 ìM, 250 ìM, and 500 ìM) also caused hUC-MSC apoptosis. This phenomenon may due to different cell origins. The authors recently found that glucose modulates metformin-induced MSC apoptosis via the AMPK-mTOR-S6k1-Bad pathway in another study. High glucose (10, 15, and 30 mmoL/L) exerts a protective effect on metformin-induced apoptosis, which is inversely proportional to the glucose level[119,120]. However, Zafarvahedian et al[114] showed that glucose conditions do not affect metformin toxicity and hyperglycemia itself inhibits the proliferation of MSCs[114]. More evidence on whether the glucose level influences metformin-induced adverse effects in MSC is needed in the future.
Metformin can affect many of these conditions by acting also through other mechanisms not related to stem cells’ actions. For example, metformin acts on liver cells to reduce liver gluconeogenesis and promote anaerobic glycolysis, on skeletal muscle cells to increase glucose uptake, and on intestinal epithelial cell to inhibit or delay gastrointestinal glucose absorption, which exerts hypoglycemic effects synergistically[1]. Metformin under physiological tolerance concentration can alleviate aging-associated inflammation via enhancing autophagy and normalizing mitochondrial function in T cells, which is a major source of inflammatory cytokines. Autophagy made it possible to prolong normal life via improving inflammation[121]. Besides, metformin can directly act on cancer cells. Liu et al[122] found that metformin can exert growth-suppressive effects on gallbladder cancer (GBC) cell lines via inhibition of p-Akt activity and the Bcl-2 family. Also, inhibition, knockdown, and upregulation of the membrane protein CLIC1 can affect GBC resistance in the presence of metformin[122]. Totally speaking, metformin exerts different effects by acting various mechanisms.
Stem cells have become a research hotspot in recent years. Many studies have applied stem cells to the fields of regenerative medicine, such as tissue engineering, and stem cell medicine such as the treatment of refractory disease stroke, autoimmune disease, neurological disease, and cardiovascular disease[16,17,55,92,123,124]. After understanding the effects and mechanisms of metformin on stem cells, we can apply metformin with stem cells in these fields to improve the therapeutic effect.
Regenerative medicine, which uses stem cell therapies to create tissues and organs and repair them, has the potential to address donor organ shortages. The seed cells (usually stem and progenitor cells) and scaffolds (biological materials) play important roles in tissue engineering. A previous study has shown that metformin in scaffolds regulates seed cell differentiation and proliferation. For example, Zhao et al[32] seeded hPDLSCs on a calcium phosphate cement scaffold delivering metformin[32]. Shahrezaee et al[124] seeded BMSCs on a polylactic acid and polycaprolactone scaffold delivering metformin and implanted the construct into calvarial bone defects in a rat model[124]. Smieszek et al[22] cocultured ADSCs with sol gel-derived silica/zirconium dioxide delivering metformin. All of the above studies found that metformin promoted osteogenic differentiation of stem cells, suggesting that metformin has the potential to promote bone tissue engineering by affecting stem cell differentiation.
Metformin and stem cells also have broad application prospects for the treatment of refractory diseases. Stroke is a public health issue, resulting in neurological disabilities in many patients. NSCs and NPCs are expected to be a new treatment for neurological disabilities. Ould-Brahim et al[17] treated a rat endothelin-1 focal ischemic stroke model with metformin-preconditioned human iPSCs (hiPSC)-NSCs. The results showed that metformin preconditioning enhanced the differentiation of hiPSC-NSCs, accelerated gross motor recovery, and reduced the infarct volume under ischemic and hypoxic conditions[17]. Metformin also can improve endothelial dysfunction and neovascularization by targeting stem and progenitor cells[5,17]. For example, metformin directly improves the function of vascular endothelial cells (ECs) and increases blood flow[125]. Furthermore, the antioxidant and anti-inflammation effects of metformin on stem cells contribute to treatment effects on neurodegenerative and cardiovascular diseases[38,54,101,102,107].
Metformin has the potential to be an anti-aging agent associated with stem cells. Adult stem cells are affected by the same aging mechanisms that involve somatic cells, resulting in an impaired regenerative capacity to effectively maintain tissues and organs[55]. For example, skeletal muscle drives human movement, and aging and atrophy of muscle are major signs of human aging. In age-related muscle atrophy (sarcopenia) and several dystrophies, regeneration cannot compensate for the loss of muscle tissue due to depletion of the satellite cell pool or the functional loss of satellite cells. Pavlidou et al[14] demonstrated that metformin delayed satellite cell activation and differentiation by favoring a quiescent, low metabolic state, thereby alleviating depletion of the satellite cell pool and the functional loss of satellite cells[14]. Moreover, the antioxidant and anti-inflammatory effects of metformin on stem cells contribute to anti-aging.
Metformin also has the potential to be an anti-cancer agent by targeting CSCs. Metformin inhibited CSCs from three aspects, including the inhibition of self-renewal and metastatic pathways, inhibition of metabolic pathways, and inhibition of chemoresistance pathways.
However, there are still some limitations and issues for the application of metformin and stem cells. For example, the effect of metformin may be different depending on the cell origin. Drug delivery is also an issue. There is still a lack of clinical research on these issues. The mechanisms and effects of metformin on myogenic differentiation and angiopoiesis of stem cells are controversial. A high glucose environment may influence cell differentiation and apoptosis[20,117]. These issues remain to be resolved in future studies.
In summary, a large number of studies have demonstrated the pleiotropic effects of metformin on stem cells. These inspiring results provide new treatment possibilities in many fields including regenerative medicine, stem cell medicine, anti-aging, and anti-cancer.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tarnawski AS S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1320] [Cited by in F6Publishing: 1196] [Article Influence: 170.9] [Reference Citation Analysis (0)] |
| 2. | Hidayat K, Du X, Wu MJ, Shi BM. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: Systematic review and meta-analysis of observational studies. Obes Rev. 2019;20:1494-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 3. | Sin HY, Kim JY, Jung KH. Total cholesterol, high density lipoprotein and triglyceride for cardiovascular disease in elderly patients treated with metformin. Arch Pharm Res. 2011;34:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Menendez JA, Vazquez-Martin A. Rejuvenating regeneration: metformin activates endogenous adult stem cells. Cell Cycle. 2012;11:3521-3522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Han F, Shu J, Wang S, Tang CE, Luo F. Metformin Inhibits the Expression of Biomarkers of Fibrosis of EPCs In Vitro. Stem Cells Int. 2019;2019:9019648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Blagosklonny MV. From rapalogs to anti-aging formula. Oncotarget. 2017;8:35492-35507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Son HJ, Lee J, Lee SY, Kim EK, Park MJ, Kim KW, Park SH, Cho ML. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:973986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Śmieszek A, Szydlarska J, Mucha A, Chrapiec M, Marycz K. Enhanced cytocompatibility and osteoinductive properties of sol-gel-derived silica/zirconium dioxide coatings by metformin functionalization. J Biomater Appl. 2017;32:570-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1692] [Cited by in F6Publishing: 1738] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 10. | Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 11. | Zhang R, Liang Q, Kang W, Ge S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol Int. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Mu W, Wang Z, Ma C, Jiang Y, Zhang N, Hu K, Li L, Wang Z. Metformin promotes the proliferation and differentiation of murine preosteoblast by regulating the expression of sirt6 and oct4. Pharmacol Res. 2018;129:462-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Marycz K, Tomaszewski KA, Kornicka K, Henry BM, Wroński S, Tarasiuk J, Maredziak M. Metformin Decreases Reactive Oxygen Species, Enhances Osteogenic Properties of Adipose-Derived Multipotent Mesenchymal Stem Cells In Vitro, and Increases Bone Density In Vivo. Oxid Med Cell Longev. 2016;2016:9785890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Pavlidou T, Marinkovic M, Rosina M, Fuoco C, Vumbaca S, Gargioli C, Castagnoli L, Cesareni G. Metformin Delays Satellite Cell Activation and Maintains Quiescence. Stem Cells Int. 2019;2019:5980465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Chung MM, Nicol CJ, Cheng YC, Lin KH, Chen YL, Pei D, Lin CH, Shih YN, Yen CH, Chen SJ, Huang RN, Chiang MC. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res. 2017;352:75-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Figueroa FE, Carrión F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Ould-Brahim F, Sarma SN, Syal C, Lu KJ, Seegobin M, Carter A, Jeffers MS, Doré C, Stanford WL, Corbett D, Wang J. Metformin Preconditioning of Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells Promotes Their Engraftment and Improves Post-Stroke Regeneration and Recovery. Stem Cells Dev. 2018;27:1085-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Pavlidou T, Rosina M, Fuoco C, Gerini G, Gargioli C, Castagnoli L, Cesareni G. Regulation of myoblast differentiation by metabolic perturbations induced by metformin. PLoS One. 2017;12:e0182475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Ma J, Zhang ZL, Hu XT, Wang XT, Chen AM. Metformin promotes differentiation of human bone marrow derived mesenchymal stem cells into osteoblast via GSK3β inhibition. Eur Rev Med Pharmacol Sci. 2018;22:7962-7968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 20. | Al Jofi FE, Ma T, Guo D, Schneider MP, Shu Y, Xu HHK, Schneider A. Functional organic cation transporters mediate osteogenic response to metformin in human umbilical cord mesenchymal stromal cells. Cytotherapy. 2018;20:650-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Sedlinsky C, Molinuevo MS, Cortizo AM, Tolosa MJ, Felice JI, Sbaraglini ML, Schurman L, McCarthy AD. Metformin prevents anti-osteogenic in vivo and ex vivo effects of rosiglitazone in rats. Eur J Pharmacol. 2011;668:477-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Smieszek A, Tomaszewski KA, Kornicka K, Marycz K. Metformin Promotes Osteogenic Differentiation of Adipose-Derived Stromal Cells and Exerts Pro-Osteogenic Effect Stimulating Bone Regeneration. J Clin Med. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Wang P, Ma T, Guo D, Hu K, Shu Y, Xu HHK, Schneider A. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12:437-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Wang YG, Qu XH, Yang Y, Han XG, Wang L, Qiao H, Fan QM, Tang TT, Dai KR. AMPK promotes osteogenesis and inhibits adipogenesis through AMPK-Gfi1-OPN axis. Cell Signal. 2016;28:1270-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Marofi F, Vahedi G, Solali S, Alivand M, Salarinasab S, Zadi Heydarabad M, Farshdousti Hagh M. Gene expression of TWIST1 and ZBTB16 is regulated by methylation modifications during the osteoblastic differentiation of mesenchymal stem cells. J Cell Physiol. 2019;234:6230-6243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Chava S, Chennakesavulu S, Gayatri BM, Reddy ABM. A novel phosphorylation by AMP-activated kinase regulates RUNX2 from ubiquitination in osteogenesis over adipogenesis. Cell Death Dis. 2018;9:754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Jia L, Xiong Y, Zhang W, Ma X, Xu X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp Cell Res. 2020;386:111717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Wu W, Ye Z, Zhou Y, Tan WS. AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells. Int J Artif Organs. 2011;34:1128-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Pt 3:607-614. [PubMed] [Cited in This Article: ] |
| 30. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3802] [Cited by in F6Publishing: 3995] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 31. | Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99:1233-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 688] [Cited by in F6Publishing: 724] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 32. | Zhao Z, Liu J, Schneider A, Gao X, Ren K, Weir MD, Zhang N, Zhang K, Zhang L, Bai Y, Xu HHK. Human periodontal ligament stem cell seeding on calcium phosphate cement scaffold delivering metformin for bone tissue engineering. J Dent. 2019;91:103220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Houshmand B, Tabibzadeh Z, Motamedian SR, Kouhestani F. Effect of metformin on dental pulp stem cells attachment, proliferation and differentiation cultured on biphasic bone substitutes. Arch Oral Biol. 2018;95:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 823] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 35. | Dadwal P, Mahmud N, Sinai L, Azimi A, Fatt M, Wondisford FE, Miller FD, Morshead CM. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Reports. 2015;5:166-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Ahn MJ, Cho GW. Metformin promotes neuronal differentiation and neurite outgrowth through AMPK activation in human bone marrow-mesenchymal stem cells. Biotechnol Appl Biochem. 2017;64:836-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Fatt M, Hsu K, He L, Wondisford F, Miller FD, Kaplan DR, Wang J. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem Cell Reports. 2015;5:988-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM. Metformin - a Future Therapy for Neurodegenerative Diseases : Theme: Drug Discovery, Development and Delivery in Alzheimer's Disease Guest Editor: Davide Brambilla. Pharm Res. 2017;34:2614-2627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 39. | Yousuf Y, Datu A, Barnes B, Amini-Nik S, Jeschke MG. Metformin alleviates muscle wasting post-thermal injury by increasing Pax7-positive muscle progenitor cells. Stem Cell Res Ther. 2020;11:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Senesi P, Montesano A, Luzi L, Codella R, Benedini S, Terruzzi I. Metformin Treatment Prevents Sedentariness Related Damages in Mice. J Diabetes Res. 2016;2016:8274689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Fu X, Zhao JX, Liang J, Zhu MJ, Foretz M, Viollet B, Du M. AMP-activated protein kinase mediates myogenin expression and myogenesis via histone deacetylase 5. Am J Physiol Cell Physiol. 2013;305:C887-C895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Tolosa MJ, Chuguransky SR, Sedlinsky C, Schurman L, McCarthy AD, Molinuevo MS, Cortizo AM. Insulin-deficient diabetes-induced bone microarchitecture alterations are associated with a decrease in the osteogenic potential of bone marrow progenitor cells: preventive effects of metformin. Diabetes Res Clin Pract. 2013;101:177-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Gao Y, Xue J, Li X, Jia Y, Hu J. Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J Pharm Pharmacol. 2008;60:1695-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Chen SC, Brooks R, Houskeeper J, Bremner SK, Dunlop J, Viollet B, Logan PJ, Salt IP, Ahmed SF, Yarwood SJ. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2017;440:57-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 45. | Kay JP, Alemzadeh R, Langley G, D'Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Molinuevo MS, Schurman L, McCarthy AD, Cortizo AM, Tolosa MJ, Gangoiti MV, Arnol V, Sedlinsky C. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010;25:211-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 47. | Bandow K, Kusuyama J, Kakimoto K, Ohnishi T, Matsuguchi T. AMP-activated protein kinase (AMPK) activity negatively regulates chondrogenic differentiation. Bone. 2015;74:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Kiefer JC. Back to basics: Sox genes. Dev Dyn. 2007;236:2356-2366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 49. | Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, Leung WK. Metformin Use and Gastric Cancer Risk in Diabetic Patients After Helicobacter pylori Eradication. J Natl Cancer Inst. 2019;111:484-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 50. | Miao ZF, Adkins-Threats M, Burclaff JR, Osaki LH, Sun JX, Kefalov Y, He Z, Wang ZN, Mills JC. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation. Cell Stem Cell 2020; 26: 910-925. e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Becerra-Bayona SM, Solarte-David VA, Sossa CL, Mateus LC, Villamil M, Pereira J, Arango-Rodríguez ML. Mesenchymal stem cells derivatives as a novel and potential therapeutic approach to treat diabetic foot ulcers. Endocrinol Diabetes Metab Case Rep. 2020;2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Shawky LM, El Bana EA, Morsi AA. Stem cells and metformin synergistically promote healing in experimentally induced cutaneous wound injury in diabetic rats. Folia Histochem Cytobiol. 2019;57:127-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Deng X, Szabo S, Chen L, Paunovic B, Khomenko T, Tolstanova G, Tarnawski AS, Jones MK, Sandor Z. New cell therapy using bone marrow-derived stem cells/endothelial progenitor cells to accelerate neovascularization in healing of experimental ulcerative colitis. Curr Pharm Des. 2011;17:1643-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Desouza CV. Does drug therapy reverse endothelial progenitor cell dysfunction in diabetes? J Diabetes Complications. 2013;27:519-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Khorraminejad-Shirazi M, Farahmandnia M, Kardeh B, Estedlal A, Kardeh S, Monabati A. Aging and stem cell therapy: AMPK as an applicable pharmacological target for rejuvenation of aged stem cells and achieving higher efficacy in stem cell therapy. Hematol Oncol Stem Cell Ther. 2018;11:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Bengal E, Perdiguero E, Serrano AL, Muñoz-Cánoves P. Rejuvenating stem cells to restore muscle regeneration in aging. F1000Res. 2017;6:76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7855] [Cited by in F6Publishing: 8837] [Article Influence: 803.4] [Reference Citation Analysis (0)] |
| 58. | Mendelsohn AR, Larrick JW. Epigenetic Age Reversal by Cell-Extrinsic and Cell-Intrinsic Means. Rejuvenation Res. 2019;22:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 59. | Fahy GM, Brooke RT, Watson JP, Good Z, Vasanawala SS, Maecker H, Leipold MD, Lin DTS, Kobor MS, Horvath S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18:e13028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 60. | Mahmoudi S, Xu L, Brunet A. Turning back time with emerging rejuvenation strategies. Nat Cell Biol. 2019;21:32-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 61. | Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016;23:1060-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 700] [Cited by in F6Publishing: 615] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 62. | Fang J, Yang J, Wu X, Zhang G, Li T, Wang X, Zhang H, Wang CC, Liu GH, Wang L. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell. 2018;17:e12765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 63. | Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 64. | Fidaleo M, Cavallucci V, Pani G. Nutrients, neurogenesis and brain ageing: From disease mechanisms to therapeutic opportunities. Biochem Pharmacol. 2017;141:63-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Na HJ, Pyo JH, Jeon HJ, Park JS, Chung HY, Yoo MA. Deficiency of Atg6 impairs beneficial effect of metformin on intestinal stem cell aging in Drosophila. Biochem Biophys Res Commun. 2018;498:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Na HJ, Park JS, Pyo JH, Jeon HJ, Kim YS, Arking R, Yoo MA. Metformin inhibits age-related centrosome amplification in Drosophila midgut stem cells through AKT/TOR pathway. Mech Ageing Dev. 2015;149:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Na HJ, Park JS, Pyo JH, Lee SH, Jeon HJ, Kim YS, Yoo MA. Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev. 2013;134:381-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 859] [Cited by in F6Publishing: 951] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 69. | Menendez JA, Joven J. Energy metabolism and metabolic sensors in stem cells: the metabostem crossroads of aging and cancer. Adv Exp Med Biol. 2014;824:117-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Liu X, Zheng H, Yu WM, Cooper TM, Bunting KD, Qu CK. Maintenance of mouse hematopoietic stem cells ex vivo by reprogramming cellular metabolism. Blood. 2015;125:1562-1565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, Foerster S, McClain CR, Chalut K, van Wijngaarden P, Franklin RJM. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell 2019; 25: 473-485. e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 72. | Serrano M. Metformin and reprogramming into iPSCs. Cell Cycle. 2012;11:1058-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14327] [Cited by in F6Publishing: 13571] [Article Influence: 848.2] [Reference Citation Analysis (0)] |
| 74. | van Leeuwen F, Frederiks F, Terweij M, De Vos D, Bakker BM. News about old histones: a role for histone age in controlling the epigenome. Cell Cycle. 2012;11:11-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Saini N, Yang X. Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin (Shanghai). 2018;50:133-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 76. | Courtois S, Durán RV, Giraud J, Sifré E, Izotte J, Mégraud F, Lehours P, Varon C, Bessède E. Metformin targets gastric cancer stem cells. Eur J Cancer. 2017;84:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 77. | Zhao B, Luo J, Wang Y, Zhou L, Che J, Wang F, Peng S, Zhang G, Shang P. Metformin Suppresses Self-Renewal Ability and Tumorigenicity of Osteosarcoma Stem Cells via Reactive Oxygen Species-Mediated Apoptosis and Autophagy. Oxid Med Cell Longev. 2019;2019:9290728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 78. | Barbieri F, Verduci I, Carlini V, Zona G, Pagano A, Mazzanti M, Florio T. Repurposed Biguanide Drugs in Glioblastoma Exert Antiproliferative Effects via the Inhibition of Intracellular Chloride Channel 1 Activity. Front Oncol. 2019;9:135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Paiva-Oliveira DI, Martins-Neves SR, Abrunhosa AJ, Fontes-Ribeiro C, Gomes CMF. Therapeutic potential of the metabolic modulator Metformin on osteosarcoma cancer stem-like cells. Cancer Chemother Pharmacol. 2018;81:49-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Deschênes-Simard X, Rowell MC, Ferbeyre G. Metformin turns off the metabolic switch of pancreatic cancer. Aging (Albany NY). 2019;11:10793-10795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Bozzi F, Mogavero A, Varinelli L, Belfiore A, Manenti G, Caccia C, Volpi CC, Beznoussenko GV, Milione M, Leoni V, Gloghini A, Mironov AA, Leo E, Pilotti S, Pierotti MA, Bongarzone I, Gariboldi M. MIF/CD74 axis is a target for novel therapies in colon carcinomatosis. J Exp Clin Cancer Res. 2017;36:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Shang D, Wu J, Guo L, Xu Y, Liu L, Lu J. Metformin increases sensitivity of osteosarcoma stem cells to cisplatin by inhibiting expression of PKM2. Int J Oncol. 2017;50:1848-1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Kim SH, Kim SC, Ku JL. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget. 2017;8:56546-56557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Zhang HH, Guo XL. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother Pharmacol. 2016;78:13-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 85. | Tan W, Tang H, Jiang X, Ye F, Huang L, Shi D, Li L, Huang X, Li L, Xie X, Xie X. Metformin mediates induction of miR-708 to inhibit self-renewal and chemoresistance of breast cancer stem cells through targeting CD47. J Cell Mol Med. 2019;23:5994-6004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 86. | Bishnu A, Sakpal A, Ghosh N, Choudhury P, Chaudhury K, Ray P. Long term treatment of metformin impedes development of chemoresistance by regulating cancer stem cell differentiation through taurine generation in ovarian cancer cells. Int J Biochem Cell Biol. 2019;107:116-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Gu X, Wang XQ, Lin MJ, Liang H, Fan SY, Wang L, Yan X, Liu W, Shen FX. Molecular interplay between microRNA-130a and PTEN in palmitic acid-mediated impaired function of endothelial progenitor cells: Effects of metformin. Int J Mol Med. 2019;43:2187-2198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6624] [Cited by in F6Publishing: 6212] [Article Influence: 230.1] [Reference Citation Analysis (1)] |
| 89. | Callaghan MJ, Ceradini DJ, Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal. 2005;7:1476-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Guo WX, Yang QD, Liu YH, Xie XY, Wang-Miao, Niu RC. Palmitic and linoleic acids impair endothelial progenitor cells by inhibition of Akt/eNOS pathway. Arch Med Res. 2008;39:434-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 543] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 92. | Ambasta RK, Kohli H, Kumar P. Multiple therapeutic effect of endothelial progenitor cell regulated by drugs in diabetes and diabetes related disorder. J Transl Med. 2017;15:185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 93. | Li WD, Du XL, Qian AM, Hu N, Kong LS, Wei S, Li CL, Li XQ. Metformin regulates differentiation of bone marrow-derived endothelial progenitor cells via multiple mechanisms. Biochem Biophys Res Commun. 2015;465:803-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Yu JW, Deng YP, Han X, Ren GF, Cai J, Jiang GJ. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc Diabetol. 2016;15:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 95. | Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 96. | Li WD, Li NP, Song DD, Rong JJ, Qian AM, Li XQ. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the AMPK/mTOR/autophagy pathway. Int J Mol Med. 2017;39:1262-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Ni HZ, Liu Z, Sun LL, Zhou M, Liu C, Li WD, Li XQ. Metformin inhibits angiogenesis of endothelial progenitor cells via miR-221-mediated p27 expression and autophagy. Future Med Chem. 2019;11:2263-2272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 98. | Asadian S, Alibabrdel M, Daei N, Cheraghi H, Maedeh Jafari S, Noshadirad E, Jabarpour M, Siavashi V, Nassiri SM. Improved angiogenic activity of endothelial progenitor cell in diabetic patients treated with insulin plus metformin. J Cell Biochem. 2018;:. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Zeng H, Jiang Y, Tang H, Ren Z, Zeng G, Yang Z. Abnormal phosphorylation of Tie2/Akt/eNOS signaling pathway and decreased number or function of circulating endothelial progenitor cells in prehypertensive premenopausal women with diabetes mellitus. BMC Endocr Disord. 2016;16:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Montazersaheb S, Kabiri F, Saliani N, Nourazarian A, Avci ÇB, Rahbarghazi R, Nozad Charoudeh H. Prolonged incubation with Metformin decreased angiogenic potential in human bone marrow mesenchymal stem cells. Biomed Pharmacother. 2018;108:1328-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, Stefanadis C. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62:667-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 102. | Chiang MC, Cheng YC, Chen SJ, Yen CH, Huang RN. Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against Amyloid-beta-induced mitochondrial dysfunction. Exp Cell Res. 2016;347:322-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 103. | Smieszek A, Kornicka K, Szłapka-Kosarzewska J, Androvic P, Valihrach L, Langerova L, Rohlova E, Kubista M, Marycz K. Metformin Increases Proliferative Activity and Viability of Multipotent Stromal Stem Cells Isolated from Adipose Tissue Derived from Horses with Equine Metabolic Syndrome. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Lin CH, Cheng YC, Nicol CJ, Lin KH, Yen CH, Chiang MC. Activation of AMPK is neuroprotective in the oxidative stress by advanced glycosylation end products in human neural stem cells. Exp Cell Res. 2017;359:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y, Zhang H, Lu L, Li C, Huang S, Xing Y, Zhou D, Meng A. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2015;87:15-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 106. | Chung MM, Chen YL, Pei D, Cheng YC, Sun B, Nicol CJ, Yen CH, Chen HM, Liang YJ, Chiang MC. The neuroprotective role of metformin in advanced glycation end product treated human neural stem cells is AMPK-dependent. Biochim Biophys Acta. 2015;1852:720-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Han Y, Yuan F, Deng C, He F, Zhang Y, Shen H, Chen Z, Qian L. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging (Albany NY). 2019;11:10252-10265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55:76-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 109. | Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 110. | Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 435] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 111. | Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, Park SH. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology (Oxford). 2020;59:1426-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Mounayar M, Kefaloyianni E, Smith B, Solhjou Z, Maarouf OH, Azzi J, Chabtini L, Fiorina P, Kraus M, Briddell R, Fodor W, Herrlich A, Abdi R. PI3kα and STAT1 Interplay Regulates Human Mesenchymal Stem Cell Immune Polarization. Stem Cells. 2015;33:1892-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662-5679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 316] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 114. | Zafarvahedian E, Roohi A, Sepand MR, Ostad SN, Ghahremani MH. Effect of metformin and celecoxib on cytotoxicity and release of GDF-15 from human mesenchymal stem cells in high glucose condition. Cell Biochem Funct. 2017;35:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 115. | Śmieszek A, Czyrek A, Basinska K, Trynda J, Skaradzińska A, Siudzińska A, Marędziak M, Marycz K. Effect of Metformin on Viability, Morphology, and Ultrastructure of Mouse Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells and Balb/3T3 Embryonic Fibroblast Cell Line. Biomed Res Int. 2015;2015:769402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Śmieszek A, Basińska K, Chrząstek K, Marycz K. In Vitro and In Vivo Effects of Metformin on Osteopontin Expression in Mice Adipose-Derived Multipotent Stromal Cells and Adipose Tissue. J Diabetes Res. 2015;2015:814896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010;9:1057-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 118. | He X, Yao MW, Zhu M, Liang DL, Guo W, Yang Y, Zhao RS, Ren TT, Ao X, Wang W, Zeng CY, Liang HP, Jiang DP, Yu J, Xu X. Metformin induces apoptosis in mesenchymal stromal cells and dampens their therapeutic efficacy in infarcted myocardium. Stem Cell Res Ther. 2018;9:306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Roohi A, Nikougoftar M, Montazeri H, Navabi S, Shokri F, Ostad SN, Ghahremani MH. High Glucose Affects the Cytotoxic Potential of Rapamycin, Metformin and Hydrogen Peroxide in Cultured Human Mesenchymal Stem Cells. Curr Mol Med. 2019;19:688-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | He X, Yang Y, Yao MW, Ren TT, Guo W, Li L, Xu X. Full title: High glucose protects mesenchymal stem cells from metformin-induced apoptosis through the AMPK-mediated mTOR pathway. Sci Rep. 2019;9:17764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM, Snyder-Cappione J, Hawk GS, Fleeman RM, Pihl RMF, Thompson K, Belkina AC, Cui L, Proctor EA, Kern PA, Nikolajczyk BS. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab 2020; 32: 44-55. e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 122. | Liu Y, Wang Z, Li M, Ye Y, Xu Y, Zhang Y, Yuan R, Jin Y, Hao Y, Jiang L, Hu Y, Chen S, Liu F, Zhang Y, Wu W, Liu Y. Chloride intracellular channel 1 regulates the antineoplastic effects of metformin in gallbladder cancer cells. Cancer Sci. 2017;108:1240-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000;7:451-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 124. | Shahrezaee M, Salehi M, Keshtkari S, Oryan A, Kamali A, Shekarchi B. In vitro and in vivo investigation of PLA/PCL scaffold coated with metformin-loaded gelatin nanocarriers in regeneration of critical-sized bone defects. Nanomedicine. 2018;14:2061-2073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 125. | Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 350] [Article Influence: 15.2] [Reference Citation Analysis (0)] |