Abstract
Introduction
Biological medicines have increased the cost of cancer treatments, which also raises concerns about sustainability. In Brazil, three monoclonal antibodies (mAbs)—bevacizumab, cetuximab, and panitumumab—are indicated for the treatment of metastatic colorectal cancer (mCRC) but not currently funded by the Unified Health System (SUS). However, successful litigation has led to funding in some cases.
Objective
Our objective was to evaluate the budgetary impact of including the mAbs bevacizumab, cetuximab, and panitumumab in standard chemotherapy for the treatment of mCRC within the SUS of Minas Gerais (MG), Brazil.
Method
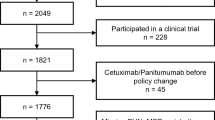
A budget impact analysis of incorporating mAbs as first-line treatment of mCRC in MG was explored. The perspective taken was that of the Brazilian SUS, and a 5-year time horizon was applied. Data were collected from lawsuits undertaken between January 2009 and December 2016, and the model was populated with data from national databases and published sources. Costs are expressed in $US.
Results
In total, 351 lawsuits resulted in funding for first-line treatment with mAbs for mCRC. The three alternative scenarios analyzed resulted in cost increases of 348–395% compared with the reference scenario. The use of panitumumab had a budgetary impact of $US103,360,980 compared with the reference scenario over a 5-year time horizon, and bevacizumab and cetuximab had budgetary impacts of $US111,334,890 and 113,772,870, respectively. The use of the anti-epidermal growth factor receptor (EGFR) mAbs (cetuximab and panitumumab) is restricted to the approximately 41% of patients with KRAS mutations, so the best cost alternative for incorporation would be the combination of panitumumab and bevacizumab, with a cost of approximately $US106 million.
Conclusion
These results highlight the appreciable costs for incorporating bevacizumab, cetuximab, and panitumumab into the SUS. Appreciable discounts are likely to be necessary before incorporation of these mAbs is approved.





Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424.
Silva ACB, Vicentini MFB, Mendoza EZ, Fujiki FK, da Fonseca LG, Braghiroli M, et al. Young-age onset colorectal cancer in Brazil: analysis of incidence, clinical features, and outcomes in a tertiary cancer center. Curr Probl Cancer. 2019;43(5):477–86.
Souza DL, Jerez-Roig J, Cabral FJ, de Lima JR, Rutalira MK, Costa JA. Colorectal cancer mortality in Brazil: predictions until the year 2025 and cancer control implications. Dis Colon Rectum. 2014;57(9):1082–9.
Guerra MR, Bustamante-Teixeira MT, Corrêa CSL, Abreu DMXd, Curado MP, Mooney M, et al. Magnitude and variation of the burden of cancer mortality in Brazil and Federation Units, 1990 and 2015. Rev Bras Epidemiol. 2017;20(Suppl 01):102–15.
Silva DAS, Tremblay MS, Souza MdFMd, Mooney M, Naghavi M, Malta DC. Mortality and years of life lost by colorectal cancer attributable to physical inactivity in Brazil (1990–2015): Findings from the Global Burden of Disease Study. PloS One. 2018;13(2):e0190943-e.
INSTITUTO NACIONAL DE CÂNCER (INCA). Estimativa 2018. Rio de Janeiro: INCA, (2018). http://www1.inca.gov.br/estimativa/2018. Accessed 18 Dec 2020.
Moghadamyeghaneh Z, Alizadeh RF, Phelan M, Carmichael JC, Mills S, Pigazzi A, et al. Trends in colorectal cancer admissions and stage at presentation: impact of screening. Surg Endosc. 2016;30(8):3604–10.
Torres OJM, Marques MC, Santos FN, Farias ICd, Coutinho AK, Oliveira CVCd, et al. Brazilian consensus for multimodal treatment of colorectal liver metastases. Module 3: controversies and unresectable metastases. Arq Bras Cir Dig. 2016;29(3):173–9.
Lopes G, Stern MC, Temin S, Sharara AI, Cervantes A, Costas-Chavarri A, et al. Early detection for colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol. 2019;5:1–22.
Atieno OM, Opanga S, Martin A, Kurdi A, Godman B. Pilot study assessing the direct medical cost of treating patients with cancer in Kenya; findings and implications for the future. J Med Econ. 2018;21(9):878–87.
Wilking N, Bucsics A, Kandolf Sekulovic L, Kobelt G, Laslop A, Makaroff L, et al. Achieving equal and timely access to innovative anticancer drugs in the European Union (EU): summary of a multidisciplinary CECOG-driven roundtable discussion with a focus on Eastern and South-Eastern EU countries. ESMO open. 2019;4(6):e000550-e.
Jakupi A, Godman B, Martin A, Haycox A, Baholli I. Utilization and expenditure of anti-cancer medicines in Kosovo: findings and implications. PharmacoEconomics Open. 2018;2(4):423–32.
Lieberman D, Gupta S. Does colon polyp surveillance improve patient outcomes? Gastroenterology. 2020;158(2):436–40.
Prasad V, Wang R, Afifi SH, Mailankody S. The rising price of cancer drugs—a new old problem? JAMA Oncol. 2016.
IQVIA Institute for Human Data Science. Global Oncology Trends 2018. https://www.iqvia.com/institute/reports/global-oncology-trends-2018. Accessed 18 Dec 2020.
Howard DH, Bach P, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139–62.
Godman B, Oortwijn W, de Waure C, Mosca I, Puggina A, Specchia ML et al. Links between pharmaceutical R&D models and access to affordable medicines. a study for the ENVI COMMITTEE. http://www.europarl.europa.eu/RegData/etudes/STUD/2016/587321/IPOL_STU(2016)587321_EN.pdf. Accessed 18 Dec 2020.
Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, et al. Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328.
Wilking N, Lopes G, Meier K, Simoens S, van Harten W, Vulto A. Can we continue to afford access to cancer treatment? Eur Oncol Haematol. 2017;13(2):114–9.
Campolina AG, Yuba TY, Decimoni TC, Leandro R, Diz MdPE, Novaes HMD, et al. Health economic evaluations of cancer in Brazil: a systematic review. Front Public Health. 2018;6(205).
Rozman LM, Campolina AG, Lopez RM, Chiba T, De Soárez PC. Palliative cancer care: costs in a Brazilian quaternary hospital. BMJ Support Palliat Care. 2019:bmjspcare-2019-001809.
da Silva MJS, O’Dwyer G, Osorio-de-Castro CGS. Cancer care in Brazil: structure and geographical distribution. BMC Cancer. 2019;19(1):987.
Santos CL, Souza AI, Figueiroa JN, Vidal SA. Estimation of the costs of invasive cervical cancer treatment in Brazil: a micro-costing study. Rev Bras Ginecol Obstet. 2019;41(6):387–93.
Ungari AQ, Pereira LRL, da Silva Castro Perdoná G, Bettim BB, Nunes AA, et al. Cost evaluation of metastatic colorectal cancer treatment in the Brazilian public healthcare system. J Integr Oncol 2015;4:136.
Caires de Souza AL, de Assis Acurcio F, Guerra Junior AA, Rezende Macedo do Nascimento RC, Godman B, Diniz LM. Insulin glargine in a Brazilian state: should the government disinvest? An assessment based on a systematic review. Appl Health Econ Health Policy. 2014;12(1):19–32.
De Oliveira GL, Guerra Junior AA, Godman B, Acurcio FA. Cost-effectiveness of vildagliptin for people with type 2 diabetes mellitus in Brazil; findings and implications. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):109–19.
Vargas-Pelaez CM, Rover MRM, Soares L, Blatt CR, Mantel-Teeuwisse AK, Rossi FA, et al. Judicialization of access to medicines in four Latin American countries: a comparative qualitative analysis. Int J Equity Health. 2019;18(1):68.
Campos Neto OH, Acurcio FdA, Machado MAdÁ, Ferré F, Barbosa FLV, Cherchiglia ML, et al. Doctors, lawyers and pharmaceutical industry on health lawsuits in Minas Gerais, Southeastern Brazil. Revista de saude publica. 2012;46(5):784–90.
Machado MAdÁ, Acurcio FdA, Brandão CMR, Faleiros DR, Guerra Jr AA, Cherchiglia ML, et al. Judicialização do acesso a medicamentos no Estado de Minas Gerais, Brasil. Revista de saude publica. 2011;45:590–8.
SECRETARIA DE ESTADO DE SAÚDE DE MINAS GERAIS (SES-MG). Judicialização da Saúde. Belo Horizonte: SES-MG (2018). http://www.saude.mg.gov.br/judicializacao. Accessed 18 Dec 2020.
Vidal TJ, Moraes EL, Retto MPF, Silva MJSd. The lawsuits to antineoplastic drugs: the tip of an iceberg? Ciencia Saude Coletiva. 2017;22(8):2539–48.
da Silva WC, de Araujo VE, Lima E, Dos Santos JBR, Silva M, Almeida P, et al. Comparative effectiveness and safety of monoclonal antibodies (Bevacizumab, Cetuximab, and Panitumumab) in combination with chemotherapy for metastatic colorectal cancer: a systematic review and meta-analysis. BioDrugs. 2018;32(6):585–606.
Carvalho AC, Leal F, Sasse AD. Cost-effectiveness of cetuximab and panitumumab for chemotherapy-refractory metastatic colorectal cancer. PloS One. 2017;12(4):e0175409-e.
Ungari AQ, Pereira LRL, Nunes AA, Peria FM. Cost-effectiveness analysis of XELOX versus XELOX plus bevacizumab for metastatic colorectal cancer in a public hospital school. BMC Cancer. 2017;17(1):691.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorect Dis. 2017;32(8):1179–90.
Marques RP, Godinho AR, Heudtlass P, Pais HL, Quintela A, Martins AP. Cetuximab versus bevacizumab in metastatic colorectal cancer: a comparative effectiveness study. J Cancer Res Clin Oncol. 2020.
Cao R, Zhang S, Ma D, Hu L. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol. 2015;32(1):325.
Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–401.
Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15(6):569–79.
Cremolini C, Antoniotti C, Lonardi S, Bergamo F, Cortesi E, Tomasello G, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the TRIBE trial by GONO. Ann Oncol. 2018;29(7):1528–34.
Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–80.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705.
Van Cutsem E, Köhne C-H, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Wagner ADADW, Arnold D, Grothey AAG, Haerting J, Unverzagt S. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. 2009(3):CD005392-CD.
Chan DLH, Segelov E, Wong RS, Smith A, Herbertson RA, Li BT, et al. Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst Rev. 2017;6(6):CD007047-CD.
Guerra-Junior AA, Pires de Lemos LL, Godman B, Bennie M, Osorio-de-Castro CGS, Alvares J, et al. Health technology performance assessment: real-world evidence for public healthcare sustainability. Int J Technol Assess Health Care. 2017;33(2):279–87.
Lemos LLP, Guerra Junior AA, Santos M, Magliano C, Diniz I, Souza K, et al. The assessment for disinvestment of intramuscular interferon beta for relapsing-remitting multiple sclerosis in Brazil. PharmacoEconomics. 2018;36(2):161–73.
COMISSÃO NACIONAL DE INCORPORAÇÃO DE TECNOLOGIAS (CONITEC). Diretrizes Metodológicas—Avaliação de desempenho de tecnologias em saúde—Desinvestimento e Reinvestimento. Brasília: Ministério da Saúde, 2016. http://conitec.gov.br/images/Consultas/2016/diretrizf_investimento_reinvestimento. Accessed 23 Sept 2020.
Maia Diniz I, Guerra AAJ, Lovato Pires de Lemos L, Souza KM, Godman B, Bennie M, et al. The long-term costs for treating multiple sclerosis in a 16-year retrospective cohort study in Brazil. PloS One. 2018;13(6):e0199446.
Faleiros DR, Alvares J, Almeida AM, de Araujo VE, Andrade EI, Godman BB, et al. Budget impact analysis of medicines: updated systematic review and implications. Expert Rev Pharmacoecon Outcomes Res. 2016;16(2):257–66.
Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
Mauskopf J, Earnshaw S. A methodological review of US budget-impact models for new drugs. PharmacoEconomics. 2016;34(11):1111–31.
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone A-M, Howlader N, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–800.
IBGE. Portal do INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA (IBGE). Projeção da população. Rio de Janeiro: IBGE, 2018. https://www.ibge.gov.br/apps. Accessed 23 Sept 2020.
van der Pool AEM, Damhuis RA, Ijzermans JNM, de Wilt JHW, Eggermont AMM, Kranse R, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorect Dis. 2012;14(1):56–61.
Ferreira-Da-Silva AL, Ribeiro RA, Santos VCC, Elias FTS, d’Oliveira ALP, Polanczyk CA. Guidelines for budget impact analysis of health technologies in Brazil. Cad Saude Publica. 2012;28(7):1223–38.
National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975–2015 (2018). https://seer.cancer.gov/archive/csr/1975_2015/index.html. Accessed 18 Dec 2020.
Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
BRASIL. Ministério da Saúde. Diretrizes metodológicas: análise de impacto orçamentário: manual para o Sistema de Saúde do Brasil. Brasília: Ministério da Saúde; 2012. http://portalarquivos2.saude.gov.br/images/pdf/2014/novembro/10/Diretrizes-metodologicas-manual-de-analise-de-impacto-orcamentario-cienciasus.pdf. Accessed 23 Sept 2020.
BRASIL. Ministério da Saúde. Oncologia: Manual de bases técnicas. 25 ed. Brasília: Ministério da Saúde; 2019. ftp://arpoador.datasus.gov.br/siasus/Documentos/APAC/Manual_Oncologia_25a_edicao.pdf. Accessed 18 Dec 2020.
Brasil. Ministério da Saúde. Manual de bases técnicas—23ª Edição. Brasilia. Ministerio da Saude; 2016. ftp://arpoador.datasus.gov.br/siasus/Documentos/APAC/Manual_Oncologia_23a_edicao.pdf. Accessed 18 Dec 2020.
Van Cutsem E, Dicato M, Arber N, Berlin J, Cervantes A, Ciardiello F, et al. Molecular markers and biological targeted therapies in metastatic colorectal cancer: expert opinion and recommendations derived from the 11th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2009. Annals of oncology. 2010;21(Suppl 6):vi1–vi10.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, Group EGW. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–iii9.
Peeters M, Kafatos G, Taylor A, Gastanaga VM, Oliner KS, Hechmati G, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials. Eur J Cancer. 2015;51(13):1704–13.
Han C-B, Li F, Ma J-T, Zou H-W. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: a meta-analysis and systematic review. Cancer Invest. 2012;30(10):741–7.
Berwick DM. Disseminating innovations in health care. JAMA. 2003;289(15):1969–75.
Sanson-Fisher RW. Diffusion of innovation theory for clinical change. Med J Austral. 2004;180(S6):S55–6.
Schneiders RE, Ronsoni RdM, Sarti FM, Nita ME, Bastos EdA, Zimmermann IR, et al. Factors associated with the diffusion rate of innovations: a pilot study from the perspective of the Brazilian Unified National Health System. Cad Saude Publica. 2016;32(9):e00067516-e.
INSTITUTO NACIONAL DE CÂNCER (INCA). Estimativa 2016. http://santacasadermatoazulay.com.br/wp-content/uploads/2017/06/estimativa-2016-v11.pdf. Accessed 23 Sept 2020.
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Santos M de O. Estimativa 2018: Incidência de Câncer no Brasil. Revista Brasileira de Cancerologia 2018;64(1):119–120.
FONTES, S. Governo autoriza reajuste anual de até 4,76% nos medicamentos. Valor Econômico. 2017. https://valor.globo.com/brasil/noticia/2017/03/31/governo-autoriza-reajuste-anual-de-ate-476-nos-medicamentos.ghtml. Accessed 18 July 2019.
Silva MT, Silva END, Pereira MG. Budget impact analysis. Epidemiol Serv Saude. 2017;26(2):421–4.
BRASIL. Ministério da Saúde. PORTARIA Nº 24, DE 24 DE ABRIL DE 2019 Torna pública a decisão de incorporar o nusinersena para atrofia muscular espinhal (AME) 5q tipo I, no âmbito do Sistema Único de Saúde—SUS. 25 de abril de 2019. Seção 1, Nº 79.
BRASIL. Ministério da Saúde. PORTARIA Nº 1.297, DE 11 DE JUNHO DE 2019. Institui projeto piloto de acordo de compartilhamento de risco para incorporação de tecnologias em saúde, para oferecer acesso ao medicamento Spinraza (Nusinersena) para o tratamento da Atrofia Muscular Espinhal (AME 5q) tipos II e III no âmbito do Sistema Único de Saúde—SUS. Diário Oficial da União Seção 1, ISSN 1677-7042 Nº 112, quarta-feira, 12 de junho de 2019a.
Zampirolli Dias C, Godman B, Gargano LP, Azevedo PS, Garcia MM, Souza Cazarim M, et al. Integrative review of managed entry agreements: chances and limitations. PharmacoEconomics. 2020.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
Rawlins MD, Chalkidou K. The opportunity cost of cancer care: a statement from NICE. Lancet Oncol. 2011;12(10):931–2.
Pontes C, Zara C, Torrent-Farnell J, Obach M, Nadal C, Vella-Bonanno P, et al. Time to review authorisation and funding for new cancer medicines in Europe? Inferences from the case of olaratumab. Appl Health Econ Health Policy. 2020;18(1):5–16.
Martin AP, Pedra G, Downing J, Collins B, Godman B, Alfirevic A, et al. Trends in BRCA testing and socioeconomic deprivation. Eur J Hum Genet. 2019;27(9):1351–60.
Ferguson JS, Summerhayes M, Masters S, Schey S, Smith IE. New treatments for advanced cancer: an approach to prioritization. Br J Cancer. 2000;83(10):1268–73.
Cohen D. Cancer drugs: high price, uncertain value. BMJ. 2017;359:j4543.
Godman B, Wild C, Haycox A. Patent expiry and costs for anti-cancer medicines for clinical use. Gener Biosimil Initiat J. 2017;6(3):105–6.
Emerich D, Viegas, F. Atrasado em 4 anos: Hospital do barreiro será entregue R$ 135 milhões mais caro. O Tempo, Contagem, 2 dez. 2015. Disponível em: https://www.otempo.com.br/cidades/hospital-do-barreiro-ser%C3%A1-entregue-r-135-milh%C3%B5es-mais-caro-1.1183195. Accessed 12 Sep 2020.
EULER Jnr. Mater Dei prepara expansão em Betim. Estado de Minas, Belo Horizonte, 11 fev. 2015. https://www.em.com.br/app/noticia/economia/2015/02/11/internas_economia,616972/mater-dei-prepara-expansao-em-betim.shtml. Accessed 11 Sep 2020.
OLIVEIRA J. À margem da crise, Unimed-BH vai investir R$ 160 mi em Betim. Hoje em dia, 12 out. 2016. https://www.hojeemdia.com.br/primeiro-plano/%C3%A0-margem-da-crise-unimed-bh-vai-investir-r-160-mi-em-betim-1.419849. Accessed 11 Sep 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Research Group on Health Economics (GPES) UFMG and is an integral part of the research projects, “Analise do impacto orçamentário no Sistema Único de Saúde (SUS) de incorporação dos medicamentos mais demandados pela via judicial nos Programas de Assistência Farmacêutica” and “Avaliação Epidemiológica, econômica e de trajetórias assistenciais de procedimentos de alto custo no SUS: utilização de base de dados centrada no paciente a partir da integração de registros dos sistemas de informação em saúde” with financial support from the National Council for Scientific and Technological Development (FAPEMIG), the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Conflicts of interest
Wânia Cristina da Silva, Brian Godman, Francisco de Assis Acúrcio, Mariângela Leal Cherchiglia, Antony Martin, Konrad Maruszczyk, Jans Bastos Izidoro, Marcos André Portella, Agner Pereira Lana, Orozimbo Henriques Campos Neto, and Eli Iola Gurgel Andrade have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Data availability
The major SUS Information Systems: Outpatient (SIA), Hospital (SIH) and Mortality (SIM), which contain approximately 3.5 million patients in cancer treatment, are not available publicly. Their access is restricted and only allowed through a data usage agreement that has a non-disclosure provision.
Author Contributions
WCS, BG, MLC, FAA, and EIGA conceived of and designed the paper and collected the data. AM, KM, and BG were involved in the writing or critical review of the paper, with important intellectual contributions and collection of some information. All authors analyzed, interpreted the data with substantial contribution, and approved the final version of the article for submission. WCS acts as guarantor that all aspects that make up the manuscript have been reviewed, discussed, and agreed among the authors in order to be exposed with maximum precision and integrity.
Rights and permissions
About this article
Cite this article
da Silva, W.C., Godman, B., de Assis Acúrcio, F. et al. The Budget Impact of Monoclonal Antibodies Used to Treat Metastatic Colorectal Cancer in Minas Gerais, Brazil. Appl Health Econ Health Policy 19, 557–577 (2021). https://doi.org/10.1007/s40258-020-00626-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00626-0