Abstract
In this work new naphthalenediimides (NDIs) were synthesized and used as dyes in DSSC. The efficiency (η) of the DSSC is influenced by NDIs electronic and structural characteristics. It was found a better cell performance with the NDIs which have a broader absorption band shifted to the red color, high ƹ values, and more adsorption in the anode surface. The band gaps were determinate by UV–vis and cyclic voltammetry. The LUMO orbitals of most of the NDIs are above of the conduction band (CB) energy for TiO2 allowing the electron transfer process from the NDI to the photoanode, especially in those with a significant LUMONDI-CBTi energetic difference. Also, NDIs with polar groups in their structure presented higher η values due to a better adsorption on the photoanode surface, which allows a better energy capture compared with those with lower adsorption.
Similar content being viewed by others
Introduction
At the present moment, solar energy is considered the most promising renewable energy source. The conversion of light is possible because of the photovoltaic effect. Many materials have been developed for capture, conversion, and transmission of light. The most studied device in the last generation is the dye-sensitized solar cells (DSSC), due to the low-cost and high efficiency in the solar to electric energy conversion [1]. DSSC comprise a variety of primary components and multiple possible combinations. The challenges are to fine-tune between each material and identify the optimal condition to improve the device performance [2]. Particularly, the dye-sensitizer play a pivotal role to ensure sufficient light capture, and it is desirable to have a sensitizer that absorbs all incident light from visible to the near-infrared region of the solar spectrum. The highest efficiency DSSC device contains metal bipyridyl sensitizers as N3 or N719 [3]. On the other hand, the efficience values for tandem and pervoskita solar solar cells are higher [4, 5]. A fundamental requirement in the development of new materials with improved properties is the challenging task of achieving a rigorous understanding of the relationship between molecular structure and charge transport properties [6]. The molecules of choice with these characteristics are the aromatic molecules which have well-defined redox and spectroscopic properties as perylenediimides, which are thermally stable and inexpensive organic molecules that are used as commercial pigments or dyes, with excellent photophysical properties like high molar absorption coefficients and fluorescence yield. These characteristics make perylenediimides ideal for light-based applications [7,8,9]. Different organic molecules with unusual electronic properties are 1,4,5,8-naphthalenediimides (NDIs) or also known as naphthalene carbodiimides. These are electron-deficient aromatic compounds capable of self-organization and have been incorporated into large multicomponent assemblies to form n-type over p-type semiconductor materials [10,11,12]. Many analogues may be synthesized by functionalization of the diimide group or substitution in the napthalene 2,3,6 and 7 positions, seeking to modulate their photophysical and physicochemical properties, change their solubility, absorption bands, and capability of form aducts or hydrogen bonds. However, the synthesis of these functionalized NDIs remains as a great challenge [13].
Continuing with our interest in the synthesis and application of NDIs, a series of six NDIs with different substituents in the imide and the naphthalene core were synthesized and tested as dyes in DSSC [14,15,16]. The photovoltaic performance of the solar cells was related to the structural, optical, and electrochemical properties of the NDIs and also to the concentration of the NDIs adsorbed onto the TiO2 surface [17, 18].
Experimental section
Materials and instrumentation
All chemical reagents were purchased from Sigma-Aldrich and were employed as received without further purification. 1,4,5,8-naphthalenetetracarboxylic dianhydride (C14H4O6, 99%), dibromoisocyanuric acid (C3HBr2N3O3, 96%), hexylamine (C6H15N, 99%), benzylamine (C7H9N, 99%), 4-methoxyphenetylamine (C9H13NO, 98%), 2-picolylamine (C6H8N2, 99%), 4-aminobenzoic acid (C7H7NO2, 99%), 4-methylaminobenzoic acid (C5H8O2, 97%), tyramine (C8H11NO, 99%), 4-aminobenzonitrile (C7H6N2, 98%), tetrabutylammonium iodide (C16H36NI, 98%), 4-tert-butylpyridine (C9H13N, 96%), iodine (I2, 99%), lithium iodide (LiI, 99%), N719 (C58H86N8O8RuS2, 95%), acetonitrile (C2H3N, 99%), tertbutanol (C4H10O, 99%) and 3-methoxypropionitrile (C4H7NO, 98%). Fluorine-doped SnO2 (TCO-30 glass, 8 Ω/sq), Plastisol T/SP (Pt commercial paste), Ti-Nanoxide T/SP (TiO2 commercial paste) and Meltonix 1170-25 (25 µm thick thermoplastic sealing film made of Surlyn®.) were purchased from Solaronix.
Spectroscopic and spectrometric characterization
1H NMR spectra were recorded using a 400 MHz Brucker Advance III NMR spectrophotometer, and the samples were dissolved in CDCl3 or DMSO-d6 containing small amounts of TMS as an internal standard. FTIR spectra were recorded using a Perkin Elmer spectrophotometer Spectrum 400 in the range of 4000 to 600 cm−1. The purity of the compounds was determined by GC–MS with an Agilent Technologies GC-Mass spectrophotometer 5975C. The optical properties of the naphthalene diimide were determined by UV–Vis in a Spectrophotometer of double beam Shimadzu model UV2700. SEM micrographs have been acquired in a JEOL JSM-7800F Schottky FE-SEM instrument to evaluate the mesoporosity of TiO2 sample, using accelerating voltage a 9.0 kV, surface roughness characterization was performed using an atomic force microscope Nanosurf EasyScan 2 in contact mode, I–V curves were characterized using a class ABB small area sun simulator 94011 A with xenon lamp and irradiance of 100 mW cm−2 coupled with a BioLogic VMP300 multichannel potentiostat.
Synthesis
2,6-Dibromo-1,4,5,6-naphthalenetetracarboxylic dianhydride (1)
The synthesis of 1 was carried out following the methodology reported by Langhals with some modifications [26]. 1,4,5,8-Naphthalenetetracarboxylic dianhydride (NDA) (0.20 g, 0.75 mmol) was placed in a Schlenk flask and dissolved in 10 mL H2SO4 at 85 °C with constant magnetic stirring. The dibromide isocyanuric acid (DBI) (0.27 g, 0.97 mmol) was added and leave to react at 120 °C for 20 h. Icy deionized water was added after the reaction time was accomplished and the reaction mixture was stirred for 2 h, and finally, the product was precipitated with methanol, filtered and used without further purification (70% yield). MS (EI) m/z: 427 (M + , 61), 382 (52), 348 (31).
Synthesis of compounds 2
The synthesis of the NDIs 2 was carried out using 0.2 g of 1 (0.472 mmol) in reflux with constant magnetic stirring for 6 h using chlorobenzene as the solvent and an excess of amine (2.83 mmol) for the complete modification in the nucleus and the imide. After the reaction time was accomplished, the solvent was removed under reduced pressure and the reaction mixture was mixed with methanol-acetone (1:1 v/v), obtaining a precipitate which was collected by filtration. Then the products were purified by flash chromatographic column. The isolated pure products were characterized by different analytical technics, such as NMR, FTIR and MS(EI). The yields were calculated based on the amount (moles) of product recovered after the purification, divided by the theoretical moles that should be obtained in the reaction.
N,N′-Dihexyl-2,6-bis(hexylamine)-1,4,5,8-naphthalenediimide (2a)
Blue solid, 0.25 g (84% yield). Mp 192 ± 2 °C. FTIR: 3276, 2956, 2926, 2856, 1677, 1622, 1586 cm−1. 1H NMR (400 MHz, CDCl3): δ 9.27 (t, J = 5.2 Hz, 2H), 8.01 (s, 2H), 4.13 (t, J = 7.6 Hz, 4H), 3.46 (dt, J = 7.6, 5.2 Hz, 4H), 2.10–1.38 (m, 32H), 0.90 (t, J = 7.2 Hz, 12H). MS(EI) m/z: 634 (M+, 100), 561 (50), 477 (25). TGA: Td = 350 ± 2 °C. ε = 19,401 Lmol−1 cm−1.
N,N′-Dibenzyl-2,6-bis(benzylamine)-1,4,5,8-naphthalenediimide (2b)
Purple solid, 0.19 g (62% yield). Mp 310 ± 2 °C. FTIR: 3310, 3065, 3031, 2963, 1690, 1632, 1583 cm−1. 1H NMR (400 MHz, CDCl3): δ 9.77 (t, J = 5.6 Hz, 2H), 8.21 (s, 2H), 7.72–7.69 (m, 8H), 7.54–7.51 (m,8H), 7.41 (m, 4H), 5.36 (brs, 4H), 4.73 (d, J = 5.6 Hz, 4H). MS(EI) m/z: 658 (M+, 17), 566, (20), 476 (15), 121 (80), 64 (100). TGA: Td = 350 ± 2 °C. ε = 14,645 Lmol−1 cm−1.
N,N′-Di(phenyl)ethyl-2,6-bis(4-methoxyphenethylamine)-1,4,5,8-naphthalenediimide (2c)
Black solid, 0.29 g, (74% yield). Mp 220 ± 2 °C. FTIR: 3250, 3072, 2961, 1682, 1659, 1630, 1581 cm−1. 1H NMR (400 MHz, DMSO-d6): δ 9.44 (t, J = 5.6 Hz, 2H), 8.16 (s, 2H), 8.72–8.69 (m, 8H), 7.54–7.51 (m, 8H), 4.35 (td, J = 7.2, 5.6 Hz, 4H), 3.73 (t, J = 7.6 Hz, 4H), 3.04 (t, J = 7.2 Hz, 4H), 2.94 (t, J = 7.6 Hz, 4H), 2.17 (s, 6H). MS(EI) m/z: 834 (M+, 18), 711, (66), 562 (30), 135 (100). TGA: Td = 398 ± 2 °C. ε = 13,294 Lmol−1 cm−1.
N,N′-Di(pyridin-2-yl)metil-2,6-bis(2-picolylamino)-1,4,5,8-naphthalenediimide (2d)
Blue solid, 0.19 g (62% yield). Mp 240 ± 2 °C. FTIR: 3249, 3038, 3007, 2924, 2853, 1680, 1636, 1581 cm−1. 1H NMR (400 MHz, CDCl3): δ 10.08 (t, J = 4.8 Hz, 2H), 8.73 (s, 2H), 8.42 (m, 2H), 8.40 (m, 2H), 7.78 (m, 2H), 7.76 (m, 2H), 7.74 (m,2H), 7.46 (m, 2H), 7.27 (m, 2H), 7.26 (m, 2H), 5.43 (brs, 8H). MS(EI) m/z: 662 (M+, 2.2), 569, (31), 478 (100), 92 (24). TGA: Td = 334 ± 2 °C. ε = 8693 Lmol−1 cm−1.
N,N′-Dihexyl-2,6-dibromo-1,4,5,8-naphthalenediimide (3)
Compound 1 (0.2 g, 0.472 mmol) was dissolved in 10 mL of glacial acetic acid under constant magnetic stirring. Then, hexylamine (0.28 g, 2.832 mmol) was added dropwise, and the mixture was refluxed for 2 h. After this time, the reaction mixture was treated with cooled methanol-acetone (1:1 v/v), obtaining a crude precipitate which was collected by filtration and purified by column chromatography using hexane-DCM (2:1 v/v). Orange solid, 0.19 g (70% yield). FTIR: 3059, 2918, 2847, 1702, 1651 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.99 (s, 2H), 4.19 (t, J = 7.6 Hz, 4H), 1.74 (qnt, J = 7.6 Hz, 4H), 1.44–1.26 (m, 12H), 0.90 (t, J = 7.2 Hz, 6H). EM(IE) m/z: 592 (M+, 100), 509 (50), 407 (25).
Synthesis of compounds 4
The synthesis of the NDIs 4 was carried out with 2 (0.47 mmol) and 2.5 molar equivalents of the amine in DMF as solvent at reflux for 12 h. After the reaction time was finished, the reaction mixture was treated with a cooled 0.1 M HCl solution to obtain a crude precipitate, which was collected by filtration and purified.
N,N′-Dihexyl-2,6-bis[4-(2-aminoethyl)phenol]-1,4,5,8-naphthalenediimide (4a)
Blue solid, 0.15 g (64% yield). Mp 124 ± 2 °C. FTIR: 3265, 3460, 3060, 2926, 2857, 1624, 1581, 1510 cm−1. 1H NMR (400 MHz, CDCl3): δ 9.54 (t, J = 5.6 Hz, 2H), 8.40 (s, 2H), 7.15 (d, J = 8.4 Hz, 4H), 6.81 (d, J = 8.4 Hz, 4H), 4.11 (m, 4H), 3.70 (td, J = 7.6, 5.6 Hz, 4H), 2.99 (t, J = 7.6 Hz, 4H) 1.75–1.35 (m, 16H), 0.90 (t, J = 6.4 Hz, 6H). MS(EI) m/z: 704 (M+), 661 (20), 647 (50), 633 (80), 619 (100). TGA: Td = 206 ± 2 °C. ε = 18,766 Lmol−1 cm−1.
N,N′-Dihexyl-2-[4-(2-aminoethyl)phenol]-1,4,5,8-naphthalenediimide (4a′)
This compound was obtained as by product in the reaction of 4a and was isolated in the soluble part in the mixture of methanol/acetone 1:1. The solvent was removed by reduced pressure. Red solid, 12% yield. Mp 220 ± 2 °C. FTIR: 3265, 3460, 3060, 2926, 2857, 1624, 1581, 1510 cm−1. 1H NMR (400 MHz, CDCl3): δ 10.13 (t, J = 5.6 Hz, 1H), 8.57 (d, J = 7.6 Hz, 1H), 8.27 (d, J = 7.6 Hz, 1H), 8.15 (s, 1H), 7.17 (d, J = 8.4 Hz, 2H), 6.81 (d, J = 8.4 Hz, 2H), 4.14 (dt, J = 7.2, 5.6 Hz, 4H), 3.76 (dt, J = 7.2, 6.0 Hz, 2H), 3.03 (t, J = 7.2 Hz, 2H), 1.72 (qnt, J = 6.0 Hz, 4H), 1.43–1.26 (m, 12H), 0.90 (t, J = 5.6 Hz, 6H). TGA: Td = 351 ± 2 °C. ε = 17,186 Lmol−1 cm−1.
N,N′-Dihexyl-2,6-bis(4-aminobenzonitrile)-1,4,5,8-naphthalenediimide (4b)
Red solid, 0.20 g (89% yield). Mp 152 ± 2 °C. FTIR: 3028, 2924, 2857, 2213, 1685, 1665, 1561 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.90 (d, J = 7.2 Hz, 4H), 8.82 (s, 2H), 8.52 (d, J = 7.2 Hz, 4H), 4.18 (m, 4H), 1.71, (m, 8H), 1.34 (m, 8H), 0.90 (t, J = 4.8 Hz, 6H). EM(IE) m/z: 666 (M+,3), 630, (100), 584 (50), 550 (92). TGA: Td = 363 ± 2 °C. ε = 14,624 Lmol−1 cm−1.
Band gap determination
The electrochemical measurements by cyclic voltammetry to calculate the NDIs band gaps were performed using a Biologic VMP-300 potentiostat/galvanostat. The experiments were carried out in a three-electrode electrochemical cell in a 0.1 M NBu4PF6 DCM solution and ferrocene at 1 × 10–3 M as an internal reference. The concentration of NDIs used for the study was of 1 × 10–3 M. In all the electrochemical studies a platinum electrode was used as working electrode. An electrode of Ag/AgCl sat. KCl and a Pt wire were utilized as reference and counter electrode, respectively. The ELUMO was calculated using the Eq. (1) and the EHOMO with the Eq. (2), where EOnset, Red is the potential in which initiates the reduction reaction (NDI/NDI−) or the potential necessary to reduce the molecule, as well as EOnset,Ox is the potential in which initiates the oxidation reaction (NDI/NDI+) or the potential necessary to oxidazed the molecule. A value of 5.1 eV, corresponding to Ferrocene system was reported in the same conditions of these experiments [19]. The band gap values estimated with UV–Vis were calculated using the Eq. (3) reported by Costa et al. [21] used on organic π conjugated systems were the λ denotes the absorption edge wavelength expressed in nm, obtained from the offset wavelength derived from the low energy absorption band of the NDIs.
DSSC fabrication
The photoanode and cathode were prepared using TCO-30 glass slides from Solaronix. TCO glass slides were cleaned by ultrasound with a detergent solution and deionized water, rinsed with milli-Q water and ethanol, followed by H2O2 at 1% treatment under UV light before anode and cathode fabrication. TiO2 blocking layer was deposited by immersion in TiCl4 aqueous solution at 0.04 M at 70 °C for 30 min; then it was rinsed with distilled water and ethanol [22]. A 0.2 cm2 mesoporous TiO2 layer was applied by doctor blade method using Ti-Nanoxide T/SP paste, and thermally treated at 375 °C for 5 min, 450 °C for 15 min, and 500 °C for 15 min, and finally treated again with TiCl4 aqueous solution and air annealed at 500 °C for 30 min. The dyes were adsorbed onto the mesoporous TiO2 layer inmersing the anode at 70 °C into a solution of 3 × 10–4 M NDI solution using DCM as solvent for 24 h. Cathodes were prepared by doctor blade method using Pt catalyzer (T/SP SOLARONIX), and air annealed at 500 °C for 30 min. The photoanode and counter electrode were then sealed together using a 25 μm thermoplastic cement (SOLARONIX DuPont Surlyn®) and filled with I−/I3− redox pair solution. The redox electrolyte constituted by a 0.05 M I2, a 0.1 M LiI, a 0.5 M 4-tert-butylpyridine and a 0.6 M tetrabutylammonium iodide solution prepared in a mixture of acetonitrile and 3-methoxypropionitrile (1:1).
Adsorption–desorption test
A calibration curve for each NDI was obtained correlating the absorbance data of NDI solutions at different concentration (1 × 10–4, 5 × 10–5, 1 × 10–5, 5 × 10–6, 1 × 10–6 and 5 × 10–7 M) in DCM. Anodes were preparated with different NDIs following the procedure detailed in the previous section, and then were heated into a muffle at 70 °C for 10 min. Later, the anodes were placed into DCM (10 mL) to desorb the NDI from the anode surface. The solutions were affored to a known volume (10 mL in case of some of the solvent was evaporate during the process) and then they were measured by UV–Vis, and the absorbance data were used to calculate the concentration of the dye in the solution.
Results and discussion
Synthesis and characterization
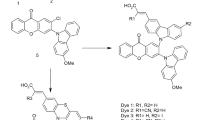
The synthesis of NDIs 2 was performed using the synthetic route shown in Scheme 1. These NDIs have the same substituent in the imide and the 2,6-naphthalene positions. 1,4,5,8-naphthalenetetracarboxylic dianhydride (NDA) was reacted with dibromoisocyanuric acid (DBI) to obtain the dibromide NDA 1 in 70% yield. Then, the dianhydride 1 was reacted with an excess of a primary amine to obtain the NDIs 2 with 62 to 84% yield. The primary amines selected for this purpose contain different structures as an alkyl (a), alkyl-aromatic (b), alkyl-substituted aromatic (c), and alkyl-heteroaromatic (d).
The synthetic route for the NDIs 4 is shown in Scheme 2. These NDIs have a hexyl substituent in the imides and a different substituent in the 2,6-naphthalene positions. The main purpose was to introduce an alkyl group in the imide to modulate the solubility in an organic solvent and to introduce several polar substituents in the naphthalene core. The dianhydride 1 was reacted with hexylamine (2.0 eq) in acetic acid for 6 h, and the dibromo-NDI 3 was obtained with 70% yield. Then the NDIs 4 were obtained by substitution of the bromines with different amines. Unfortunately, only NDIs 4a and 4b could be isolated as pure compounds. The yields were 62 and 89%, respectively. From the purification process of the crude of 4a, a small amount of the monosubstituted NDI 4a′ was isolated as pure compound. All the NDIs showed high solubility in DCM and DMSO.
In the characterization by FTIR the vibrations for the symmetric and asymmetric stretching of carbonyl are observed around 1660 cm−1 and the flexing vibration of the amine groups around 1500 cm−1 (Figures S1–S7). The 1H NMR characterization of the NDIs shows the characteristic triplet signal above at 9.0 ppm corresponding to the two amine hydrogens and a singlet around 8.4 ppm that integrate for the two hydrogens of the naphthalene core (Figures S8–S15). The rest of the signals correspond to the substituents present in the NDI. The melting and decomposition temperatures of the NDIs were determinate using thermogravimetric analysis (TGA), the temperatures ranging from 206 to 398 °C showing high thermal stability. There is only one loss of weighing for NDIs 2 (80%), while in NDIs 4, the thermic decomposition occurs in three steps, taking the first weightloss as the degradation point (Figure S16).
Optical properties of NDI dyes
The absorption bands of NDIs have a relative maximum absorption peak located in the range of 362–372 nm, due to the typical π–π* transition. A second absorption peak is present from 532 to 616 nm, corresponding to auxochrome n–π* transition of the amino-substituents in the naphthalene. As can be seen in Fig. 1, the absorption band of NDIs 2 is red-shifted with minimal differences in their maximum peaks and bandwidth, covering a wide area of the visible spectrum from 400 to 670 nm, which is a desirable characteristic for a dye application. The effect of the substituents was mainly observed in the molar absorptivity coefficient (Ƹ). The NDI 2a has hexyl substituents and presents the highest value, while 2d having pyridylmethyl substituents has the lowest (Table 1). NDI 4a has similar spectral properties than NDIs 2, but it has higher absorptivity throughout the entire spectrum. NDI 4a′ with one substituent in the naphthalene, has a maximum absorption peak shifted to blue at 527 nm, and its absorption band is narrower than for 4a (Figure S17). NDI 4b has a cyanophenyl substituent directly attached to the naphthalene, and its absorption band is shifted to blue with a maximum absorbance peak at 532 nm, this difference is attributed to the electron-withdrawing groups in the phenyl rings, affecting the electron delocalization toward the naphthalene. The Ƹ of NDIs 4 are in the same order (104) and are close to that for 2a, which may indicate that the presence of the alkyl substituent in the imide favorably affects the Ƹ. On the other hand, the Ƹ of N719 and “Black dye” are 14,700 and 7000, respectively [3], all the NDIs from this work have higher Ƹ value than the “Black dye” and similar or highest values than N719.
Band gaps of NDIs
The band gaps estimated from the HOMO and LUMO energies (eV) measured by cyclic voltammetry and by UV–Vis spectroscopy are presented in Table 1. Ferrocene was used as a reference system in the cyclic voltammetry analysis [19]. The quasi-reversible oxidation and reduction peaks belonging to the NDIs (1 × 10–3 M) were observed in the cyclic voltammetry in DCM (Figure S18–S24). The first peak at positive potential corresponds to the NDI/NDI+ oxidation process and the second peak to the NDI+/NDI2+ process. However, the latest oxidation is not reversible due to electron donating groups attached to the naphthalene stabilize the positive charge [20]. At negative potentials the first peak corresponds to a NDI/NDI− reduction process to form an anionic radical, a second peak is observed at higher potential corresponding to NDI−/NDI2− process to form a dianion. Noteworthy, 2d does not shows an oxidation peak (Figure S21); this may due to the interference with the ferrocene system, the NDI react oxidizing the ferrocene, while 2d is reduced [21]. The band gaps calculated from the UV–Vis spectra have a difference of 0.01 and 0.18 eV with respect to the electrochemical values, giving a reasonable estimation of the real value. The values of 2d and 4a′ were estimated only by UV–Vis from the onset wavelength of the absorption band ACN because of in the electrochemical analysis some lateral reactions between ferrocene and the reduced NDI take place (Figure S22) [22]. According to the TiO2 CB values reported in the literature (− 4.20 eV, [23, 24] − 4.00 eV, [25] − 4.05 eV, [26] and − 4.00 eV [27]), the LUMO energy of the most of NDIs are above of TiO2 CB allowing the electronic transfer from the NDI to TiO2 (Figure S4), but exists the possibility of potential barriers at the TiO2/NDIs interphase, that can limit the injection of photogenerated charge carriers into the mesoporous Electron Transport Layer (ETL), inducing recombination process, and cutting down the generated current. On the other hand, the HOMO orbitals of the NDIs (− 6.54 to − 5.79 eV) above of the TiO2 valence band (VB, − 7.40 eV) facilititates the cross over between systems and the injection of holes from the TiO2 VB to the NDI HOMO orbital increasing the charge trapping and the photogenerated current (Figure S25) [23].
Effect of NDI concentration over DSSC photovoltaic parameters
An important factor in DSSC performance, which is not commonly studied, is the amount or concentration (mass/area) of adsorbed dye in the anode surface. The amount of adsorbed dye depends on different factors such as the stock solution concentration and the adsorption time, among others [28]. Here, the surface of TiO2 was first studied without dye by SEM and EDS (Figure S26). The SEM micrograph of TiO2 shows some aggregates at a macro scale due to the manual deposition method, but in a magnification of 100 nm, it can be observe a homogeous distribution of the particles. The EDS analysis shows a high concentration of oxygen in the analyzed surface of TiO2, which favors the adsorption of dye molecules by hydrogen bonding and the formation of some adducts [29].
Then, anodes sensitized with different stock NDI solutions in DCM at 3 × 10–4 M for 24 h were prepared. Meanwhile, calibration curves for each NDI were obtained correlating the maximum absorbance of NDI solutions at different concentration in DCM. The anodes were heated into a muffle at 70 °C for 10 min and then were sumerged into DCM to desorb the NDI from the anode surface. The resultant solutions were affored to a known volume and analyzed by UV–Vis. The absorbance value was used to calculate the concentration of dye in the solution, and therefore, the amount of desorbed NDI.
Based on the concentration value of dye stock solutions and the concentration of dye desorbed solutions, it was found that the adsorption percentages of NDI were in the range of 0.12 to 2.98% and the adsorbed concentration in 5.55 × 10–6 to 2.34 × 10–4 mmol/cm2 (Table 2). As can be seen, the adsorbed concentration onto TiO2 is different for each NDI regardless the stock solution had the same concentration. The photovoltaic parameter values are not directly related with the concentration. For example, solar cells sensitized with 2d and 4a show similar efficiency but their concentrations are ten-times different. Noteworthy, all the solar cells sensitized with NDIs containing polar groups and larger absorption wavelengths show the higher ƞ values. The concentration values obtained with these NDIs are in the same magnitude of those reported in literature for other dyes as N719 [30].
A detailed study of dye concentration and photovoltaic parameters was done varying the sensitization time at 3, 6, 12, 18, and 24 h, as well as testing the adsorption–desorption processes. NDIs 4a, 4a′ and 2d were selected for the study due to they offered the best performance in the preliminary studies. NDIs 4a and 4a′ induce a visible change in the anode color, the intensity increases proportionally to the exposure time (3–12 h), but later, the color intensity decreases (18 and 24 h) (Figure S27). It is possible that the NDI staked and detached at high concentration from the anodic surface during the experiment. This is congruent with the results listed in Table 3, the concentration of NDI in the anodic surface increases from 3 to 12 h, but after a long period of sensitization the concentration of NDIs decreases. Unlike, the absortion of 2d is higher and faster, the concentration of 2d in the anode is one order of magnitude higher than 4a and 4a′, and apparently the interaction with the anode surface is stronger because of it requires higher time to achieve the desorption.
The solar cells sensitized with 4a′ and 4a have a maximun or a peak of efficience at an optimum concentration (Figure S28 and Table S1), the efficience decreases mainly due to a staking effect at higher concentration. This effect is more significant in 4a′ than 4a, probably due to a major steric impediment or hindrance in the latter (it has two R groups), which does not allow the staking. The solar cells sensitized with 4a′ reach a maximum efficience and Jsc with 9.54 × 10–6 mmol cm−2 and 4a reaches the highest values at 2.19 × 10–5 mmol cm−2. A different behavior is observed in cells sensitized with 2d, the efficience and the current density increase directly to the concentration, which is the usual behavior of the most of dyes in solar cells.
The anode surface was analyzed by atomic force microscopy before and after the dye adsorption (Fig. 2). A slight increase in the surface roughness (roughness data Sa) was generated by the adsorption of 4a. A smooth surface was observed in both cases without the presence of agglomerations that could act as traps or recombination centers confirming the improvement of fill factor due maximization of shunt resistance [31].
Photovoltaic performance of DSSC sensitized with NDIs
The analysis of all DSSC indicates that the positioning of the energy level of NDIs versus the electrolyte is independent from the concentration of NDI onto the surface, so the Voc for all cases is 408 ± 6 mV. For those solar cells, where NDI concentration is higher (2.5–3.2 × 10–5 mmol cm−2) similar values of filling factor are observed, thus the low photocurrents are attributed to recombination processes that now take place by the transfer of the photogenerated electron between the dye molecules that are stacked, and from there to the ETL.
The J–V curves presented in Fig. 3 show the characteristic slopes caused by a high series resistance (Rs) and moderate shunt resistance (Rsh), which results in low filling factors due to recombination processes in the transference of photogenerated charge. As observed in Table 3, the DSSC sensitized with 4a and 2d show the highest Jsc values (2.81 and 2.36 mA cm−2, respectively). The current generated using 4a as dye is due to an optimal concentration of dye adsorbed on the mesoporous electron transport layer, but it is possibly limited by a charge recombination in the TiO2/NDI. Another possible limitant in the electronic transfer through the high generation of charge carriers (due to high dye concentration) is the recombination processes that take place between dye molecules. The 2d sensitized solar cell has higher photovoltage although their LUMO level positioning is more electropositive than 4a. The origin of photovoltage has not yet been fully understood, Voc is most likely related with the difference between the Fermi level of semiconductor electrode (now coupled with LUMO level of dye) and the electrolyte redox potencial. However, the largest potential difference is limited by the energy level of HOMO and LUMO of the sensitizer dye. Based on the irradiance spectrum of sun simulator xenon lamp, it is possible to calculate the number of carriers(photons) that touch the surface of the solar cell and pass through the absorber layers generating electron–hole pairs, this number can be useful to determine the maximum current density that can be obtained from a photovoltaic device absorbing 100% of the photons with energy larger than the band gap calculated for each absorber. In the case of the analyzed NDIs 2d and 4a, the ratio between measured current density from the cell and the theoretical current density was 0.183 and 0.202, repectively.
Figure 4 shows the J–V curves for 4a sensitized solar cells obtained at different sensitization time. The results show that the optimum concentration of 4a on the anodic surface is 2.19 × 10–5 mmol cm−2 and allows to obtain the maximum value of current density of 2.81 mA cm−2 at three hours of sensitization. This is corroborated analyzing the J–V curve generated by the sensitized cell at 24 h, which has a 4a concentration of 1.53 × 10–5 mmol cm−2 and JSC of 1.53 mA cm−2.
Influence of NDIs subtituents over the DSSC performace
The previous results show that polar groups in the NDIs substituents have a significant influence in the photovoltaic performance, possibly the polarization of the electronic density in the dye allows the electronic transfer. For example, comparing solar cells sensitized with 2a (hexyl substituents) and 4a ((hydroxyphenyl)ethyl substituents), both NDIs have a broad absorption band (from 450 to 650 nm), and their ƹ and band gaps are similar. However, the concentration of 4a in the anode is less than the half of 2a, and there is a significant difference in the DSSC performance, which is attributed to a more efficient injection of electrons into TiO2 and less recombination with 4a.
The different electronic conjugation in 4a and 4b due to a different connectivity of the substituent to the naphthalene core has an important effect over the photovoltaic performance. The electronic conjugation of along the whole molecule and the electronwithdrawing effect of cyano generate a narrower absorption band (400 and 600 nm) than the observed with 4a (400 and 680 nm). It is observed that the JSC and the η decrease in the 4b sensitized solar cell probably due to electron-withdrawing effect of cyano groups which decrease the electron transfer to the surface of the photoanode. The electronic conjugation is interrupted by the spacer in 4a, then the availability of the electrons to delocalized in the naphthalene core and to be transferred to the photoanode surface are better, and therefore, the JSC increases.
On the other hand, the substituents number in the naphthalene core influence the DSSC performance. As an example, the solar cells sensitized with 4a (disubstituted in positions 2 and 6) and 4a′ (monosubstituted) at the same concentration, have similar filling factors, but 4a solar cell generates four times more JSC and have three times more η than 4a′. The presence of two-electron donor groups in the naphthalene enriches the electronic density favouring the electron promotion to the anode surface. Also, the electronic absorption band for 4a is more extensive than for 4a′, and it is shifted to the red (Figure S17), then it is expected more capture of solar radiation with 4a.
Nevertheless, there are some factors affecting the performance of the solar cells sensitized with NDIs. The well-known formation of stable negative radicals may inhibits the charge diffusion to the anode. NDI 4a has the lowest value of band gap calculated from the electrochemical experiments, in which case is easy to transfer electrons from the HOMO orbital to the LUMO orbital generating anionic radicals and affecting the performance of the cells at high concentrations of the NDI. On the other hand, even 2d has the highest concentration of dye in the anodic surface, it has the lowest ε and the highest band gap value (calculated by UV–Vis spectroscopy), requiring a high amount of solar energy and concentration to generate charge carriers.
Despite these limitations, 4a has the best characteristics as a dye in DSSC due to several electronic and structural characteristics that make it suitable for this application, and may helpful in the design of new NDIs for this purpose. NDI 4a has two substituents with hydroxyl groups, which promotes the adsorption of the molecule on the photoanode porous surface, it has a broad UV–vis absorption band shifted to the red, and its ƹ is high compared with the other NDIs. The band gap of 4a is one of the narrowest calculated for the NDIs in the study, and its HOMO and LUMO energy levels are substantially above of the VB and CB for TiO2, and the maximum efficiency is reached at low concentration of dye adsorbed onto the anode without affecting the roughness of the surface. All these features improve the DSSC performance sensitized with 4a.
Finally, there are several reports in the literature related with NDIs used in DSSC and BHJ solar cells [32, 33]. The η values obtained with the NDIs studied in this work are comparable or even superior with those NDIs previously reported, despite the differences in FF or the working area (Table 4). Another important aspect is the simple molecular structure of this NDIs indicating the viability of the synthesized molecules as dyes in solar cells.
Conclusions
The structure and the physicochemical properties of the NDIs used as dyes simultaneously influence the performance of the solar cells. From this point of view, the NDI properties that positively affect the DSSC performance are the presence of polar groups to ensure the dye adsorption in the photoanode surface, the number of electron-donor substituents and connectivity in the naphthalene core, because these modulate the position and widening of the electronic absorption bands. Also, the ƹ, HOMO and LUMO energies, as well as the band gaps are affected by the chemical structure of the substituents. All these features together must be considerer to improve the performance of NDIs as dyes in solar cells.
References
Bella, F., Gerbaldi, C., Barolo, C., Grätzel, M.: Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 44, 3431–3473 (2015)
Gong, J., Sumathy, K., Qiao, Q., Zhou, Z.: Review on dye-sensitized solar cells (DSSCs): advanced techniques and research trends. Renew. Sustain. Energy Rev. 68, 234–246 (2017)
Ren, Y., Sun, D., Cao, Y., Tsao, H.N., Yuan, Y., Zakeeruddin, S.M., Wang, P., Grätzel, M.: A stable blue photosensitizer for color palette of dye-sensitized solar cells reaching 12.6% efficiency. J. Am. Chem. Soc. 140, 2405–2408 (2018)
Adachi, D., Hernández, J.L., Yamamoto, K.: Impact of carrier recombination on fill factor for large area heterojunction crystalline silicon solar cell with 25.1% efficiency. Appl. Phys. Lett. 107, 233506 (2015)
Sahli, F., Werner, J., Kamino, B.A., Bräuninger, M., Monnard, R., Paviet-Salomon, B., Barraud, L., Ding, L., Diaz-Leon, J.J., Sacchetto, D., Cattaneo, G., Despeisse, M., Boccard, M., Nicolay, S., Jeangros, Q., Niesen, B., Ballif, C.: Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 17, 820–826 (2018)
Kar, S., Sizochenko, N., Ahmed, L., Batista, V.S., Leszczynski, J.: Quantitative structure-property relationship model leading to virtual screening of fullerene derivatives: exploring structural attributes critical for photoconversion efficiency of polymer solar cell acceptors. Nano Energy 26, 677–691 (2016)
Kolhe, B.N., Dev, R.N., Satyaprasad, P., Jancy, B., Narayan, K.S., Asha, S.K.: Structure engineering of naphthalene diimides for improved charge carrier mobility: self-assembly by hydrogen bonding, good or bad? J. Mater. Chem. 22, 15235–15246 (2012)
Bhosale, S.-V., Jami, C.-H., Langford, J.-S.: Chemistry of naphthalene diimides. Chem. Soc. Rev. 37, 331–342 (2008)
Ferrere, S., Gregg, A.B.: New perylenes for dye sensitization of TiO2. New J. Chem. 26, 1155–1160 (2002)
Saeed, U., Bibi, S., Rehman, S., Rauf-Khan, S., Shoaib, M., Iqbal, M.A., Nawaz, F., Jia, R.: Designation and match of non-fullerene acceptors with X-shaped donors toward organic solar cells. ChemistrySelect 4, 3654–3664 (2019)
Wu, W., Zhao, Z., Li, J., Chen, M., Gao, X.: Photoredox catalysis in organophosphorus chemistry. Asian J. Org. Chem. 10, 1002 (2018)
Suraru, S.L., Zschieschang, U., Klauk, H., Würthner, F.: A core-extended naphthalene diimide as a p-channel semiconductor. Chem. Commun. 47, 11504–11506 (2011)
Würthner, F., Ahmed, S., Thalacker, C., Debaerdemaeker, T.: Core‐substituted naphthalene bisimides: new fluorophors with tunable emission wavelength for FRET studies. J. Chem. Eur. 8, 4742–4750 (2002)
Ramírez-Zatarain, S.D., Ochoa-Terán, A., Reynoso-Soto, E.A., Miranda-Soto, V., Félix-Navarro, R.M., Pina-Luis, G., Yatsimirsky, A.K.: Selective interaction of N, N-bis (aminobenzyl) naphthalenediimides with fluoride anion. Supramol. Chem. 29, 446–454 (2017)
Martínez-Quiroz, M., Ochoa-Terán, A., Pina-Luis, G., Santacruz-Ortega, H.: Photoinduced electron transfer in N, N-bis (pyridylmethyl) naphthalenediimides: study of their potential as pH chemosensors. Supramol. Chem. 29, 32–39 (2017)
Alatorre-Barajas, J.A., Ramírez-Zatarain, S.D., Ochoa-Terán, A., Cordova, J., Reynoso-Soto, E.A., Chávez, D., Miranda-Soto, V., Labastida-Galván, V., Ordoñez, M.: An efficient method for the synthesis of new non‐symmetrical naphthalenediimides. ChemistrySelect 3, 11943–11949 (2018)
Boucléa, J., Ackermann, J.: Solid‐state dye‐sensitized and bulk heterojunction solar cells using TiO2 and ZnO nanostructures: recent progress and new concepts at the borderline. Polym. Int. 61, 355–373 (2012)
Susarova, K.D., Troshin, A.P., Höglinger, D., Koeppe, R., Babenko, D.S., Lyubovskaya, N.R., Razumov, F.V., Sariciftci, S.N.: Donor–acceptor complex formation in evaporated small molecular organic photovoltaic cells. Sol. Energy Mater. Sol. Cells. 94, 803–811 (2010)
Cardona, C.M., Li, W., Kaifer, A.E., Stockdale, D., Bazon, G.C.: Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 23, 2367–2371 (2011)
Thalacker, C., Röger, C., Würthner, F.: Synthesis and optical and redox properties of core-substituted naphthalene diimide dyes. J. Org. Chem. 71, 8098–8105 (2006)
Costa, J.C.S., Taveira, R.J.S., Lima, C.F.R.A.C., Mendes, A., Santos, L.N.B.F.: Optical band gaps of organic semiconductor material. Opt. Mater. 58, 51–60 (2016)
Xu, Q., Wang, J.S., Li, C.W., Wang, L.H.: Transition metal nitrides for electrochemical energy applications. Express Polym. Lett. 7, 842–851 (2013)
Zhu, R., Jiang, C.Y., Liu, B., Ramakrishna, S.: Highly efficient nanoporous TiO2‐polythiophene hybrid solar cells based on interfacial modification using a metal‐free organic dye. Adv. Mater. 21, 994–1000 (2009)
Bouclea, J., Ackermann, J.: Solid‐state dye‐sensitized and bulk heterojunction solar cells using TiO2 and ZnO nanostructures: recent progress and new concepts at the borderline. Polym. Int. 61, 355–373 (2012)
Marques, F.C., Jasieniak, J.J.: Ionization potential and electron attenuation length of titanium dioxide deposited by atomic layer deposition determined by photoelectron spectroscopy in air. Appl. Surf. 422, 504–508 (2017)
Fujisawa, J., Eda, T., Hanaya, M.: Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 685, 23–26 (2017)
Long, R., Dai, Y., Meng, G., Huang, B.: Energetic and electronic properties of X-(Si, Ge, Sn, Pb) doped TiO2 from first-principles. Chem. Phys. 11, 8165–8172 (2009)
Tang, X., Wang, Y., Cao, G.: Effect of the adsorbed concentration of dye on charge recombination in dye-sensitized solar cells. J. Electroanal. Chem. 694, 6–11 (2013)
Ooyama, Y., Inoue, S., Asada, R., Ito, G., Kushimoto, K., Komaguchi, K., Imae, I., Harima, Y.: Direct arylation of benzo[b]furan and other benzo-fused heterocycles. Eur. J. Org. Chem. 1, 91–100 (2010)
Murakami, T.M., Grätzel, M.: Optically transparent cathode for dye-sensitized solar cells based on graphene nanoplatelets. Inorg. Chem. Acta 361, 572–580 (2008)
Ma, Z., Tang, Z., Wang, E., Andersson, M.R., Inganäs, O., Zhang, F.: Influences of surface roughness of ZnO electron transport layer on the photovoltaic performance of organic inverted solar cells. J. Phys. Chem. C 116, 24462–24468 (2012)
Niu, H., Mu, J., Zhang, M., Luo, J., Luo, P., Bai, X., Wang, W.: Naphthalene-containing polyimides: synthesis, characterization and photovoltaic properties of novel donor-acceptor dyes used in solar cell. Trans. Nonferrous Met. Soc. China 19, s587–s593 (2009)
Roshan, F., Zhenghao, M., Evan, M., Sauvé, G.: Tuning the organic solar cell performance of acceptor 2, 6-dialkylaminonaphthalene diimides by varying a linker between the imide nitrogen and a thiophene group. J. Phys. Chem. 118, 3433–3442 (2014)
Acknowledgements
Authors thank to Tecnológico Nacional de México (TecNM) (Grant no. 5859.16-P) and Consejo Nacional de Ciencia y Tecnología (CONACyT-México) (Grant no. PN-2015-92) for the financial support to this research. C. L. Castro-Riquelme thanks CONACyT for the postgraduate scholarship. Authors are grateful to Supramolecular Chemistry Thematic Network (Grant no. RTQS 294810) for their support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castro-Riquelme, C.L., Ochoa-Terán, A., Calva-Yáñez, J.C. et al. New naphthalenediimides as potential dyes for TiO2-sensitized solar cells. Mater Renew Sustain Energy 10, 2 (2021). https://doi.org/10.1007/s40243-021-00190-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-021-00190-0