Abstract
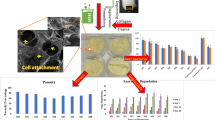
Skin acts as protective barrier against a number of factors such as dust, opportunistic microbial and viral infections, regulates body temperature and waste discharge. Fibroblast cell population plays an important role in development of skin architecture. A scaffold having capability to support and enhance fibroblast growth is a viable option for wound dressing material which can shorten the time for wound to heal. In this work, Silk Fibroin (SF) and Xanthan (Xa) were blended in three ratios 80 SF: 20 Xa (SFX82), 60 SF: 40 Xa (SFX64), and 50 SF: 50 Xa (SFX55) to create SF/Xa scaffold. Miscibility and other physicochemical properties of SF/Xa scaffold are functions of blending ratios and blend with the ratio 80 SF: 20 Xa has the highest miscibility. Thermal properties of SF/Xa blends are a function of miscibility with SFX82 having superior thermal properties of all fabricated scaffolds. The porosity of SF/Xa scaffolds is in the range of 67% to 50%, with pore size of 58.1 µm–45.5 µm, water uptake capacity of 92%–86%, and surface roughness of 49.95 nm–385 nm. SFX82 shows highest growth rate of L929 fibroblast cells indicating its superiority over other scaffolds for providing biological cues for the growth and proliferation of fibroblastic cells in natural environment. SFX82 scaffold is found to be most suitable for fibroblastic cells thereby enhancing the tissue regeneration at wound site.
Similar content being viewed by others
References
De Moraes M A, Beppu M M. Biocomposite membranes of sodium alginate and silk fibroin fibers for biomedical applications. Journal of Applied Polymer Science, 2013, 130, 3451–3457.
Sorrel J M, Caplan A I. Fibroblast heterogeneity: More than skin deep. Journal of Cell Science, 2004, 117, 667–675.
Desimone M F, Helary C, Rietveld I B, Bataille I, Mosser G, Guille-Guirad M M, Livage J, Coradin T. Silica-collagen bionanocomposites as three-dimensional scaffolds for fibroblast immobilization. Acta Biomaterilia, 2010, 6, 3998–4004.
Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A, Reis R L, Kaplan D L, Kundu S C. Silk fibroin for skin injury repair: Where do things stand?. Advanced Drug Delivery Reviews, 2019, 153, 28–53.
Kundu B, Kurland N E, Bano S, Patra C, Engel F B, Yadavalli V K, Kundu S C. Silk proteins for biomedical applications: Bioengineering perspectives. Progress in Polymer Science, 2014, 39, 251–267.
Szewczyk P K, Stachewicz U. Collagen fibers in crocodile skin and teeth: A morphological comparison using light and scanning electron microscopy. Journal of Bionic Engineering, 2020, 17, 669–676.
Pawelska-Kaczmarek A. Alginate-based hydrogels in regenerative medicine. In Leonel Pereira ed., IntechOpen, Alginates — Recent Uses of This Natural Polymer, 2019, 1–16.
Li M, Li H C, Li X G, Zhu H, Xu Z H, Liu L Q, Ma J J, Zhang M J. A bioinspired alginate-gum arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing. ACS Applied Material Interfaces, 2017, 9, 22160–22175
Kundu B, Rajkhowa R, Kundu S C, Wang X. Silk fibroin biomaterials for tissue regenerations. Advanced Drug Delivery Review, 2013, 65, 457–470.
Wang Y, Bella E, Lee C S D, Migliaresi C, Pelcastre L, Schwartz Z, Boyan B D, Motta A. The synergistic effects of 3-D porous silk fibroin matrix scaffold properties and hydrodynamic environment in cartilage tissue regeneration. Biomaterials, 2010, 31, 4672–4681.
Omenetto F G, Kaplan D L. New opportunities for an ancient material. Science, 2010, 329, 528–531
Faria S, Petkowicz C L D O, Morais S A L D, Terrones M G H T, Resende M M, de Franca F P, Cardoso V L. Characterization of xanthan gum produced from sugar cane broth. Carbohydr Polym, 2011, 86, 469–476.
Shera S S, Sahu S, Banik R M. Preparation of drug eluting natural composite scaffold using response surface methodology and artificial neural network approach. Tissue Engineering and Regenerative Medicine, 2018, 15, 131–143
Zhao G, Cui R, Chen Y, Zhou S, Wang C, Hu Z H, Zheng X, Li M O, Qu S U. 3D printing of well dispersed electrospun PLGA fiber toughened calcium phosphate scaffolds for osteoanagenesis. Journal of Bionic Engineering, 2020, 17, 652–668.
Ling S, Qi Z M, Knight D P, Shao Z, Chen X. FTIR imaging: A useful method for studying the compatibility of silk fibroin-based polymer blends. Polymer Chemistry, 2013, 4, 5401–5406.
Kim U J, Park J, Kim H J, Wada M, Kaplan D L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials, 2005, 26, 2775–2785.
Pal K, Banthia A K, Majumdar D K. Biomedical evaluation of polyvinyl alcohol — gelatin esterified hydrogel for wound dressing. Journal of Material Science: Material in Medicine, 2007, 18, 1889–1894.
Kirdponpattara S, Khamkeaw A, Sanchavanakit N, Pavasant P, Phisalaphong M. Structural modification and characterization of bacterial cellulose-alginate composite scaffolds for tissue engineering. Carbohydrate Polymer, 2015, 132, 146–155.
Wu S O, Deng L, Hsia H, Xu K I, He Yu, Huang Q, Peng Y, Zhou Z, Peng C. Evaluation of gelatin-hyaluronic acid composite hydrogels for accelerating wound healing. Journal of Biomaterials Applications, 2017, 31, 1380–1390.
Wang H Y, Zhang Y Q. Processing and characterisation of a novel electropolymerized silk fibroin hydrogel membrane. Scientific Reports, 2014, 4, 6182.
Saleh H M, Annuar M S M, Simarani K. Ultrasound degradation of xanthan polymer in aqueous solution: Its scission mechanism and the effect of NaCl incorporation. Ultrasonics Sonochemistry, 2017, 39, 250–261.
Pei Y, Liu X, Liu S, Lu Q A, Liu J, Kaplan D L, Zhu H. A mild process to design silk scaffolds with reduced β-sheet structure and various topographies at the nanometer scale. Acta Biomaterilia, 2015, 13, 168–176.
Mao C F, Rwei S P. Cascade analysis of mixed gels of xanthan and locust bean gum. Polymer, 2006, 47, 7980–7987.
Gobin A S, Froude V E, Mathur A B. Structural and mechanical characteristics of silk fibroin and chitosan blend scaffolds for tissue regeneration. Journal of Biomedical Material Research A, 2005, 74, 465–473.
He J X, Wang Y, Cui S, Gao Y, Wang S. Structure and properties of silk fibroin/carboxymethyl chitosan blend films. Polymer Bulletin, 2010, 65, 395–409.
Zhou J, Cao C, Ma X L, Lin J. Electrospinning of silk fibroin and collagen for vascular tissue engineering. International Journal of Biological Macromolecules, 2010, 47, 514–519.
Zhang F, Zuo B Q, Zhang H X. Studies of electrospun regenerated SF/TSF nanofibers. Polymer, 2009, 50, 279–285.
Weska R F, Vieira W C, Nogueira G M, Beppu M M. Effect of freezing methods on the properties of lyophilized porous silk fibroin membranes. Materials Research, 2009, 12, 233–237.
Yan S, Li M Z, Zhang Q, Wang J. Blend films based on silk fibroin/hyaluronic acid. Fibers and Polymers, 2013, 14, 188–194.
Zhu J, Shao H, Hu X H. Morphology and structure of electrospun mats from regenerated silk fibroin aqueous solutions with adjusting pH. International Journal of Biological Macromolecules, 2007, 41, 469–474.
Moradi S, Hadjesfandiari N, Toosi S F, Kizakkedathu N J, Hatzikiriakos S G. Effect of extreme wettability on platelet adhesion on metallic implants: From super hydrophilicity to super hydrophobicity. ACS Applied Material Interfaces, 2016, 8, 17631–17641.
Basal G, Bayraktar O. Antibacterial properties of silk fibroin/chitosan blend films loaded with plant extract. Fibers and Polymers, 2010, 11, 21–27.
Lungan M, Popa M, Racovita S Hitruc G, Doroftei F, Desbrieres J, Vasiliu S. Surface characterization and drug release from porous microparticles based on methacrylic monomers and xanthan. Carbohydrate Polymer, 2015, 125, 323–333.
Raposo M, Ferreira Q, Ribeiro P. A guide for atomic force microscopy analysis of soft condensed matter. In Méndez-Vilas A and Díaz J eds., Modern Research and Educational Topics in Microscopy, 2007, 758–769.
Liu L, Ercan B, Sun L, Ziemer K S, Webster T J. Understanding the role of polymer surface nanoscale topography on inhibiting bacteria adhesion and growth. Biomaterial Science and Engineering, 2016, 2, 122–130.
Ai F I, Li H G, Wang Q, Yuan W J, Chen X, Yang L, Zhao J, Zhang Y. Surface characteristics and blood compatibility of PVDF/PMMA membranes. Journal of Material Science, 2012, 47, 5030–5040.
Zhu A, Zhang M, Wu J N, Shen J. Covalent immobilization of chitosan/heparin complex with a photosensitive hetero-bifunctional crosslinking reagent on PLA surface. Biomaterials, 2002, 23, 4657–4665.
Acknowledgment
The first author thanks School of Biochemical Engineering, Indian Institute of Technology (Banaras Hindu University) and Ministry of Human Resource and Development, Government of India, for providing financial support regarding for carrying out the present research work. Authors also thank Central Instrument Facility Centre (CIFC), IIT (BHU) for providing characterization facilities and Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology, Bombay, for FTIR imaging and Thermal analysis facility.
Author information
Authors and Affiliations
Corresponding author
Supplementary file
42235_2021_4_MOESM1_ESM.pdf
Development of Tunable Silk Fibroin/Xanthan Biopolymeric Scaffold for Skin Tissue Engineering Using L929 Fibroblast Cells
Rights and permissions
About this article
Cite this article
Shera, S.S., Banik, R.M. Development of Tunable Silk Fibroin/Xanthan Biopolymeric Scaffold for Skin Tissue Engineering Using L929 Fibroblast Cells. J Bionic Eng 18, 103–117 (2021). https://doi.org/10.1007/s42235-021-0004-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-021-0004-4