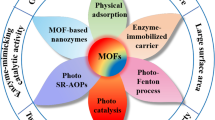
Abstract
Rapid industrialization is deteriorating air and water quality by exposing life to a wide range of pollutants, thus calling for efficient and affordable remediation strategies. Metal–organic frameworks (MOFs) are emerging materials for environmental remediation applications due to their high surface area, ordered porous structure, and application-specific tailoring of properties. In particular, transition metal-based frameworks are advanced adsorbents and catalysts for the remediation of organic and gaseous pollutants. Physicochemical properties are mainly dependent on the choice of the metal center, the oxidation state, and organic linkers. Bimetallic-, polyoxometalate-, and metal oxide-incorporated frameworks find applications as photocatalysts for decontamination of dyes, phenolic compounds, pesticides and pharmaceutical drugs under ultraviolet (UV)/visible radiations. Large surface area coupled with high activity of transition metal frameworks allows the capture and removal of inorganic and volatile organic pollutants. Transition metal frameworks convert gaseous pollutants into value-added chemicals. Frameworks containing synthetic and natural fibers are currently studied to remove chemical warfare agents.











Similar content being viewed by others
Abbreviations
- AgIO3/MIL-53 (Fe):
-
Fe-based MOF composited with AgIO3
- BiOBr/UiO-66:
-
Zr-based MOF combined with BiOBr
- CdBDC:
-
Cadmium 1,4-benzenedicarboxylate
- Co2Cl2(BBTA):
-
Co2Cl2(1H,5H-benzo(1,2-d),(4,5-d′)bistriazole)
- Co2Cl2(btdd)(H2O)2 :
-
Co2Cl2(bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo [1,4]dioxin)(H2O)2
- Co–DMOF–TM:
-
Co2(1,4-benzenedicarboxylate)2(1,4-diazabicyclo[2.2.2]octane)
- CPL-1:
-
Cu2(2,3-pyrazinedicarboxylate)2(pyrazine)
- CTAB-modified UiO-66:
-
Cetyltrimethylammonium bromide-modified UiO-66
- Cu2Cl2(BBTA):
-
Cu2Cl2(1H,5H-benzo(1,2-d),(4,5-d′)bistriazole)
- Cu2O@SiO2 :
-
Silica-coated copper oxide(I) nanocages
- Cu2O@ZIF-8:
-
Cu2O loaded within ZIF-8
- Cu-BDC(ted)0.5 :
-
Cu(1,4-benzenedicarboxylate)(triethylenediamine)0.5
- CuBDC:
-
Copper 1,4-benzenedicarboxylate
- CuBTC:
-
Copper benzene-1,3,5-tricarboxylate
- CuBTC-P:
-
Non-thermal plasma-treated CuBTC
- D-M-Fe:
-
Fe-based MOF (MIL-53 (Fe)) modified with D-sorbitol
- Fe3O4@MOF-2:
-
Zn-based MOF-supported Fe3O4 nanoparticles
- Fe3O4@SiO2 :
-
Magnetite nanoparticles coated with silica
- Fe3O4@SiO2@Zn–TDPAT:
-
Zn-based MOF with TDPAT loaded with Fe3O4@SiO2
- Fe3O4-COOH@ZIF-8/Ag/Ag3PO4 :
-
Fe3O4 with carboxylate and Ag/Ag3PO4 nanoparticles
- Fe-BTC:
-
Iron benzene-1,3,5-tricarboxylate
- Fe-M MOFs:
-
Bimetallic Fe-based MOF, M: transition metal
- Fe-Zn BDC BMOF:
-
Iron zinc bimetallic MOF with BDC
- HKUST-1:
-
Cu-based MOF using trimesic acid
- HPU-5:
-
{[Co(5-(3,5-Di-pyridin-4-yl-[1,2,4]triazol-1-ylmethyl)-isophthalic acid)](CH3CN)0.5(H2O)5}n
- HPU-6:
-
{[Mn2((5-(3,5-Di-pyridin-4-yl-[1,2,4]triazol-1-ylmethyl)-isophthalic acid))2(H2O)2](CH3OH)2(H2O)3(DMA)2}
- IRMOF-3:
-
Zn4O(2-aminoterephthalate)3
- KAUST-7:
-
Ni(NbOF5)(pyrazine)2·2H2O
- KAUST-8:
-
[Ni(AlF5(OH2))(pyrazine)2·2H2O
- LED:
-
Light-emitting diode
- M.MIL-100(Fe)@ZnO NS:
-
Fe (III)-based MOF modified with ZnO nanospheres
- MFM-520:
-
Zn2(4,4-bipyridyl-3,3′,5,5′-tetracarboxylate)
- MFM-601:
-
Zr6(μ3-O)4(μ3-OH)4(OH)4(H2O)4(4,4′,4″,4′′′-(1,4-Phenylenebis(pyridine-4,2,6-triyl))tetrabenzoate)2
- MIL(Fe)/Fe-SPC:
-
Fe-doped nanospongy porous biocarbon (SPC) composited with Fe-based MOF
- MIL-100(Fe):
-
Fe3(H2O)3O[1,3,5-benzenetricarboxylate]2·nH2O
- MIL-101(Cr):
-
Cr3F(H2O)2O[benzene dicarboxylate]3·nH2O
- MIL-101(Fe):
-
Fe3O(H2O)2Cl(1,4-benzenedicarboxylate)3
- MIL-101-NH2(Cr):
-
Cr3F(H2O)2O[2-aminoterephthalate]3·nH2O
- MIL-125(Ti)@TiO2 :
-
Ti based MOF with encapsulated TiO2
- MIL-125(Ti):
-
Ti8O8(OH)4(1,4-benzenedicarboxylate)6
- MIL-47(V):
-
VIV(O)(1,4-benzenedicarboxylate)
- MIL-53(Fe):
-
[Fe(OH).(benzene dicarboxylate).H2O]
- Mn2Cl2(btdd)(H2O)2 :
-
Mn2Cl2(bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo[1,4]dioxin)(H2O)2
- MOF 1:
-
3D-[Zn4(μ4-O)(6-oxo-6,7-dihydro-5H-dibenzo[d,f][1,3]-diazepine-3,9-dicarboxylate)3]
- MOF 3:
-
[Zn2(6-oxo-6,7-dihydro-5H-dibenzo[d,f][1,3]-diazepine-3,9-dicarboxylate)2(4,4′-bipyridine)]
- MOF 4:
-
[Zn2(6-oxo-6,7-dihydro-5H-dibenzo[d,f][1,3]-diazepine-3,9-dicarboxylate)2(1,2-bis(4-pyridyl)ethane)]
- MOF:
-
Metal-organic framework
- MOF-177:
-
Zn4O(4,4′,4′′-benzene-1,3,5-triyltribenzoate)2
- MOF-5:
-
Zn4O(1,4-benzenedicarboxylate)3
- MOF-74(Zn):
-
Zn2(2,5-dihydroxyterephthalate)
- MOF-808:
-
Zr6O4(OH)4(benzene-1,3,5-tricarboxylate)2
- NH2-MIL-125(Ti):
-
Ti8O8(OH)4(2-aminoterephthalate)6
- Ni(bdc)(ted)0.5 :
-
Ni(1,4-benzenedicarboxylate)(triethylenediamine)0.5
- Ni2Cl2(BBTA):
-
Ni2Cl2(1H,5H-benzo(1,2-d),(4,5-d′)bistriazole)
- Ni2Cl2(btdd)(H2O)2 :
-
Ni2Cl2(bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo [1,4]dioxin)(H2O)2
- Ni–DMOF–TM:
-
Ni2(1,4-benzenedicarboxylate)2(1,4-diazabicyclo[2.2.2]octane)
- NU-1000:
-
Zr6(μ3-OH)8(OH)8(1,3,6,8-tetrakis(p-benzoic acid)pyrene)2
- NU‐1401:
-
Zr6(μ3‐O)4(μ3‐OH)4(HCOO)4(OH)4(H2O)4(C30H10N2O12)
- PCN-222(Zr):
-
Zr6(μ3-O)8(OH)8(tetrakis(4-carboxyphenyl)porphyrin)2
- PMo12@UiO-66@H2SMIPs:
-
H3PMo12O40@UiO-66@ H2S molecular imprinted polymers
- PW@HKUST-1H3PW12O40·nH2O:
-
Keggin-type polyoxometalate encapsulated in CuBTC MOF
- TDPAT:
-
2,4,6-tris(3,5-dicarboxy phenylamino)-1,3,5-triazine
- TiO2@NH2-MIL-88B(Fe):
-
Iron-based MOF with TiO2 grafted on the surface
- TiO2@NH2-UiO-66:
-
Zr-based MOF with encapsulated TiO2
- UiO-66/g-C3N4/Ag(15):
-
Zr-based MOF modified with graphite carbonitride and silver nanoparticles
- UiO-66:
-
Zr6O4(OH)4(1,4-benzenedicarboxylate)6
- UiO-66-NH2 :
-
Zr6O4(OH)4(2-aminobenzenedicarboxylate)6
- UiO-67:
-
Zr6O4(OH)4(biphenyl-4,4’-dicarboxylate)6
- UiO-68-TBTD:
-
Triazolobenzothiadiazole-Zr6O4(OH)4(p,p’-terphenyldicarboxylate)6
- UMCM-313:
-
Zr6(μ3-O)4(μ3-OH)4(OH)4(H2O)4(2,5,8,11-tetrakis(4-carboxyphenyl)perylene)2
- WO3/MIL-53(Fe):
-
Fe-based MOF combined with WO3
- XY-M-Fe:
-
Fe-based MOF (MIL-53 (Fe)) modified with xylitol
- ZIF-67:
-
Co(2-methylimidazole)2
- ZIF-8:
-
Zn(2-methylimidazole)2
- Zn(bdc)(ted)0.5 :
-
Zn(1,4-benzenedicarboxylate)(triethylenediamine)0.5
- ZnBDC:
-
Zinc 1,4-benzenedicarboxylate
- Zn–DMOF–TM:
-
Zn2(1,4-benzenedicarboxylate)2(1,4-diazabicyclo[2.2.2]octane)
- Zn–TDPAT:
-
Zn-based MOF with TDPAT
- α-Fe2O3@MIL-101(Cr)@TiO2 :
-
Cr-based MOF modified with α-Fe2O3 and TiO2-modified ZIF-8
- α-Fe2O3@UiO-66:
-
UiO-66 MOF loaded with hematite nanoclusters
- 30UiO-66/CdIn2S4 :
-
Zr-based MOF combined with mixed metal sulfide (CdIn2S4)
- 1-NO3-OH·20H2O:
-
Cu9(OH)6Cl2(1-imidazol-1-yl-3-(1,2,4-triazol-4-yl)propane)6(bdc)3](NO3)2(OH)2
References
Abazari R, Mahjoub AR (2018) Amine-functionalized Al-MOF#@ xy Sm2O3–ZnO: A visible light-driven nanocomposite with excellent photocatalytic activity for the photo-degradation of amoxicillin. Inorg Chem 57:2529–2545. https://doi.org/10.1021/acs.inorgchem.7b02880
Abdpour S, Kowsari E, Moghaddam MRA (2018) Synthesis of MIL-100(Fe)@MIL-53(Fe) as a novel hybrid photocatalyst and evaluation photocatalytic and photoelectrochemical performance under visible light irradiation. J Solid State Chem 262:172–180. https://doi.org/10.1016/j.jssc.2018.03.018
Agrawal M, Sava Gallis DF, Greathouse JA, Sholl DS (2018) How useful are common simulants of chemical warfare agents at predicting adsorption behavior? J Phys Chem C 122:26061–26069. https://doi.org/10.1021/acs.jpcc.8b08856
Ahmad M, Chen S, Ye F et al (2019) Efficient photo-Fenton activity in mesoporous MIL-100(Fe) decorated with ZnO nanosphere for pollutants degradation. Appl Catal B Environ 245:428–438. https://doi.org/10.1016/j.apcatb.2018.12.057
Ahmed S, Rasul MG, Martens WN et al (2010) Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 261:3–18. https://doi.org/10.1016/j.desal.2010.04.062
Alivand MS, Shafiei-Alavijeh M, Tehrani NHMH et al (2019) Facile and high-yield synthesis of improved MIL-101(Cr) metal-organic framework with exceptional CO2 and H2S uptake; the impact of excess ligand-cluster. Microporous Mesoporous Mater 279:153–164. https://doi.org/10.1016/j.micromeso.2018.12.033
Alvaro M, Carbonell E, Ferrer B et al (2007) Semiconductor behavior of a metal-organic framework (MOF). Chem-A Eur J 13:5106–5112. https://doi.org/10.1002/chem.200601003
Azhar MR, Vijay P, Tadé MO et al (2018) Submicron sized water-stable metal organic framework (bio-MOF-11) for catalytic degradation of pharmaceuticals and personal care products. Chemosphere 196:105–114. https://doi.org/10.1016/j.chemosphere.2017.12.164
Bahri M, Haghighat F, Kazemian H, Rohani S (2016) A comparative study on metal organic frameworks for indoor environment application: Adsorption evaluation. Chem Eng J 313:711–713. https://doi.org/10.1016/j.cej.2016.10.004
Bariki R, Majhi D, Das K et al (2020) Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl Catal B Environ 270:118882. https://doi.org/10.1016/j.apcatb.2020.118882
Bayliss PA, Ibarra IA, Pérez E et al (2014) Synthesis of metal-organic frameworks by continuous flow. Green Chem 16:3796–3802. https://doi.org/10.1039/c4gc00313f
Bedia J, Muelas-Ramos V, Peñas-Garzón M et al (2019) A review on the synthesis and characterization of metal organic frameworks for photocatalytic water purification. Catalysts 9:52. https://doi.org/10.3390/catal9010052
Belarbi H, Gonzales P, Basta A, Trens P (2019) Comparison of the benzene sorption properties of metal organic frameworks: Influence of the textural properties. Environ Sci Process Impacts 21:407–412. https://doi.org/10.1039/c8em00481a
Benito J, Sorribas S, Lucas I et al (2016) Langmuir-Blodgett films of the metal-organic framework MIL-101(Cr): Preparation, characterization, and CO2 adsorption study using a QCM-based setup. ACS Appl Mater Interfaces 8:16486–16492. https://doi.org/10.1021/acsami.6b04272
Brandt P, Nuhnen A, Lange M et al (2019) Metal-organic frameworks with potential application for SO2 separation and flue gas desulfurization. ACS Appl Mater Interfaces 11:17350–17358. https://doi.org/10.1021/acsami.9b00029
Buru CT, Platero-Prats AE, Chica DG et al (2018) Thermally induced migration of a polyoxometalate within a metal-organic framework and its catalytic effects. J Mater Chem A 6:7389–7394. https://doi.org/10.1039/c8ta02562b
Buru CT, Wasson MC, Farha OK (2020) H5PV2Mo10O40 polyoxometalate encapsulated in NU-1000 metal-organic framework for aerobic oxidation of a mustard gas simulant. ACS Appl Nano Mater 3:658–664. https://doi.org/10.1021/acsanm.9b02176
Cao J, Yang ZH, Xiong WP et al (2018) One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem Eng J 353:126–137. https://doi.org/10.1016/j.cej.2018.07.060
Carter JH, Han X, Moreau FY et al (2018) Exceptional adsorption and binding of sulfur dioxide in a robust zirconium-based metal-organic framework. J Am Chem Soc 140:15564–15567. https://doi.org/10.1021/jacs.8b08433
Chandra R, Singh V, Tomar S, Nath M (2019) Multi-core-shell composite SnO2NPs@ZIF-8: potential antiviral agent and effective photocatalyst for waste-water treatment. Environ Sci Pollut Res 26:23346–23358. https://doi.org/10.1007/s11356-019-05646-5
Chen DM, Zhang XJ (2019) A polyoxometalate template metal-organic framework with unusual {Cu8(μ4-OH)6}10+ secondary building unit for photocatalytic dye degradation. Inorg Chem Commun 108:107523. https://doi.org/10.1016/j.inoche.2019.107523
Chen DM, Liu XH, Zhang NN et al (2018a) Immobilization of polyoxometalate in a cage-based metal-organic framework towards enhanced stability and highly effective dye degradation. Polyhedron 152:108–113. https://doi.org/10.1016/j.poly.2018.05.059
Chen Y, Zhang F, Wang Y et al (2018b) Recyclable ammonia uptake of a MIL series of metal-organic frameworks with high structural stability. Microporous Mesoporous Mater 258:170–177. https://doi.org/10.1016/j.micromeso.2017.09.013
Chen J, Chao F, Ma X et al (2019a) Synthesis of flower-like CuS/UiO-66 composites with enhanced visible-light photocatalytic performance. Inorg Chem Commun 104:223–228. https://doi.org/10.1016/j.inoche.2019.04.022
Chen Z, Ma K, Mahle JJ et al (2019b) Integration of metal-organic frameworks on protective layers for destruction of nerve agents under relevant conditions. J Am Chem Soc 141:20016–20021. https://doi.org/10.1021/jacs.9b11172
Chen J, Zhang X, Bi F et al (2020a) A facile synthesis for uniform tablet-like TiO2/C derived from Materials of Institut Lavoisier-125(Ti) (MIL-125(Ti)) and their enhanced visible light-driven photodegradation of tetracycline. J Colloid Interface Sci 571:275–284. https://doi.org/10.1016/j.jcis.2020.03.055
Chen L, Wang HF, Li C, Xu Q (2020b) Bimetallic metal-organic frameworks and their derivatives. Chem Sci 11:5369–5403. https://doi.org/10.1039/d0sc01432j
Chen SS, Hu C, Liu CH et al (2020c) De Novo synthesis of platinum-nanoparticle-encapsulated UiO-66-NH2 for photocatalytic thin film fabrication with enhanced performance of phenol degradation. J Hazard Mater 397:122431. https://doi.org/10.1016/j.jhazmat.2020.122431
Chen Y, Du Y, Liu P et al (2020d) Removal of ammonia emissions via reversible structural transformation in M(BDC) (M=Cu, Zn, Cd) metal-organic frameworks. Environ Sci Technol 54:3636–3642. https://doi.org/10.1021/acs.est.9b06866
Cheng M, Lai C, Liu Y et al (2018) Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord Chem Rev 368:80–92. https://doi.org/10.1016/j.ccr.2018.04.012
Cheng J, Hu T, Li W et al (2020) Stable zinc metal-organic framework materials constructed by fluorenone carboxylate ligand: multifunction detection and photocatalysis property. J Solid State Chem 282:121125. https://doi.org/10.1016/j.jssc.2019.121125
Chun H, Yizhong W, Hongxiao T (2000) Destruction of phenol aqueous solution by photocatalysis or direct photolysis. Chemosphere 41:1205–1209. https://doi.org/10.1016/S0045-6535(99)00539-1
Chung KT (2016) Azo dyes and human health: a review. J Environ Sci Heal-Part C Environ Carcinog Ecotoxicol Rev 34:233–261. https://doi.org/10.1080/10590501.2016.1236602
Clarivate Accelerating innovation (2019) Web of Science. https://login.webofknowledge.com/error/Error?Error=IPError&PathInfo=%2F&RouterURL=https%3A%2F%2Fwww.webofknowledge.com%2F&Domain=.webofknowledge.com&Src=IP&Alias=WOK5. Accessed 31 May 2020
De Andrade JR, Oliveira MF, Da Silva MGC, Vieira MGA (2018) Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: a review. Ind Eng Chem Res 57:3103–3127. https://doi.org/10.1021/acs.iecr.7b05137
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167:1–9. https://doi.org/10.1016/j.jhazmat.2008.12.114
Deng Y, Vellingiri K, Kim KH et al (2018) Activation strategies of metal-organic frameworks for the sorption of reduced sulfur compounds. Chem Eng J 350:747–756. https://doi.org/10.1016/j.cej.2018.06.006
DeSantis D, Mason JA, James BD et al (2017) Techno-economic analysis of metal-organic frameworks for hydrogen and natural gas storage. Energy Fuels 31:2024–2032. https://doi.org/10.1021/acs.energyfuels.6b02510
Dhakshinamoorthy A, Asiri AM, García H (2016) Metal-organic framework (MOF) compounds: photocatalysts for redox reactions and solar fuel production. Angew Chemie-Int Ed 55:5414–5445. https://doi.org/10.1002/anie.201505581
Diercks CS, Liu Y, Cordova KE, Yaghi OM (2018) The role of reticular chemistry in the design of CO2 reduction catalysts. Nat Mater 17:301–307. https://doi.org/10.1038/s41563-018-0033-5
Du PD, Thanh HTM, To TC et al (2019) Metal-organic framework MIL-101: synthesis and photocatalytic degradation of remazol black B dye. J Nanomater 2019:6061275. https://doi.org/10.1155/2019/6061275
Dwyer DB, Lee DT, Boyer S et al (2018) Toxic organophosphate hydrolysis using nanofiber-templated UiO-66-NH2 metal-organic framework polycrystalline cylinders. ACS Appl Mater Interfaces 10:25794–25803. https://doi.org/10.1021/acsami.8b08167
Fang XD, Yang LB, Dou AN et al (2018) Synthesis, crystal structure and photocatalytic properties of a Mn(II) metal-organic framework based on a thiophene-functionalized dicarboxylate ligand. Inorg Chem Commun 96:124–127. https://doi.org/10.1016/j.inoche.2018.08.017
Farzaneh F, Mehraban Z, Norouzi F (2010) Synthesis and utilization of a mesoporous nickel oxide as a novel catalyst for degradation of phenol. Environ Chem Lett 8:69–72. https://doi.org/10.1007/s10311-008-0193-7
Feng S, Wang R, Feng S et al (2019) Synthesis of Zr-based MOF nanocomposites for efficient visible-light photocatalytic degradation of contaminants. Res Chem Intermed 45:1263–1279. https://doi.org/10.1007/s11164-018-3682-8
Flügel EA, Ranft A, Haase F, Lotsch BV (2012) Synthetic routes toward MOF nanomorphologies. J Mater Chem 22:10119–10133. https://doi.org/10.1039/c2jm15675j
Gangu KK, Maddila S, Mukkamala SB, Jonnalagadda SB (2016) A review on contemporary metal-organic framework materials. Inorganica Chim Acta 446:61–74. https://doi.org/10.1016/j.ica.2016.02.062
Gao Y, Yu G, Liu K et al (2017) Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework. Chem Eng J 330:157–165. https://doi.org/10.1016/j.cej.2017.06.139
Gascon J, Corma A, Kapteijn F, Llabres i Xamena FX (2014) Metal organic framework catalysis: quo vadis? ACS Catal 4:361–378. https://doi.org/10.1002/chin.201414255
Geisse AR, Ngule CM, Genna DT (2019) Removal of lead ions from water using thiophene-functionalized metal-organic frameworks. Chem Commun 56:237–240. https://doi.org/10.1039/c9cc09022c
Gil A, Assis FCC, Albeniz S, Korili SA (2011) Removal of dyes from wastewaters by adsorption on pillared clays. Chem Eng J 168:1032–1040. https://doi.org/10.1016/j.cej.2011.01.078
Glomb S, Woschko D, Makhloufi G, Janiak C (2017) Metal-organic frameworks with internal urea-functionalized dicarboxylate linkers for SO2 and NH3 adsorption. ACS Appl Mater Interfaces 9:37419–37434. https://doi.org/10.1021/acsami.7b10884
Gómez-Avilés A, Peñas-Garzón M, Bedia J et al (2019) Mixed Ti-Zr metal-organic-frameworks for the photodegradation of acetaminophen under solar irradiation. Appl Catal B Environ 253:253–262. https://doi.org/10.1016/j.apcatb.2019.04.040
Gong Q, Liu Y, Dang Z (2019a) Core-shell structured Fe3O4@GO@MIL-100(Fe) magnetic nanoparticles as heterogeneous photo-Fenton catalyst for 2,4-dichlorophenol degradation under visible light. J Hazard Mater 371:677–686. https://doi.org/10.1016/j.jhazmat.2019.03.019
Gong X, Zhao R, Qin J et al (2019b) Ultra-efficient removal of NO in a MOFs-NTP synergistic process at ambient temperature. Chem Eng J 358:291–298. https://doi.org/10.1016/j.cej.2018.09.222
Gong X, Shu Y, Jiang Z et al (2020) Metal-organic frameworks for the exploitation of distance between active sites in efficient photocatalysis. Angew Chemie-Int Ed 59:5326–5331. https://doi.org/10.1002/anie.201915537
Guo X, Geng S, Zhuo M et al (2019) The utility of the template effect in metal-organic frameworks. Coord Chem Rev 391:44–68. https://doi.org/10.1016/j.ccr.2019.04.003
Gupta VK, Ali I, Saini VK et al (2005) Removal of dyes from wastewater using bottom ash. Ind Eng Chem Res 44:3655–3664. https://doi.org/10.1021/ie0500220
Gupta A, Viltres H, Gupta NK (2020a) Sono-adsorption of organic dyes onto CoFe2O4/Graphene oxide nanocomposite. Surf. Interfaces 20:100563. https://doi.org/10.1016/j.surfin.2020.100563
Gupta NK, Ghaffari Y, Bae J, Kim KS (2020b) Synthesis of coral-like α-Fe2O3 nanoparticles for dye degradation at neutral pH. J Mol Liq 301:112473. https://doi.org/10.1016/j.molliq.2020.112473
Gupta NK, Ghaffari Y, Kim S et al (2020c) Photocatalytic degradation of organic pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) nanoparticles at neutral pH. Sci Rep 10:4942. https://doi.org/10.1038/s41598-020-61930-2
Han Y, Zhang L, Bai C et al (2018) Fabrication of AgI/MIL-53(Fe) composites with enhanced photocatalytic activity for rhodamine B degradation under visible light irradiation. Appl Organomet Chem 32:1–8. https://doi.org/10.1002/aoc.4325
Han X, Yang S, Schr M (2019) Porous metal-organic frameworks as emerging sorbents for clean air. Nat Rev Chem 3:108–118. https://doi.org/10.1038/s41570-019-0073-7
Hao OJ, Kim H, Chiang PC (2000) Decolorization of wastewater. Crit Rev Environ Sci Technol 30:449–505. https://doi.org/10.1080/10643380091184237
Harvey JA, McEntee ML, Garibay SJ et al (2019) Spectroscopically resolved binding sites for the adsorption of sarin gas in a metal-organic framework: insights beyond Lewis acidity. J Phys Chem Lett 10:5142–5147. https://doi.org/10.1021/acs.jpclett.9b01867
Hassan MM, Carr CM (2018) A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 209:201–219. https://doi.org/10.1016/j.chemosphere.2018.06.043
He L, Dong Y, Zheng Y et al (2019) A novel magnetic MIL-101(Fe)/TiO2 composite for photo degradation of tetracycline under solar light. J Hazard Mater 361:85–94. https://doi.org/10.1016/j.jhazmat.2018.08.079
He X, Chen DR, Wang WN (2020) Bimetallic metal-organic frameworks (MOFs) synthesized using the spray method for tunable CO2 adsorption. Chem Eng J 382:122825. https://doi.org/10.1016/j.cej.2019.122825
Howarth AJ, Peters AW, Vermeulen NA et al (2017) Best practices for the synthesis, activation, and characterization of metal-organic frameworks. Chem Mater 29:26–39. https://doi.org/10.1021/acs.chemmater.6b02626
Hu Q, Di J, Wang B et al (2019) In-situ preparation of NH2 -MIL-125(Ti)/BiOCl composite with accelerating charge carriers for boosting visible light photocatalytic activity. Appl Surf Sci 466:525–534. https://doi.org/10.1016/j.apsusc.2018.10.020
Huang Y, Wang R (2019) Highly selective separation of H2S and CO2 using a H2S-imprinted polymers loaded on a polyoxometalate@Zr-based metal-organic framework with a core-shell structure at ambient temperature. J Mater Chem A 7:12105–12114. https://doi.org/10.1039/c9ta01749f
Huang W, Jing C, Zhang X et al (2018) Integration of plasmonic effect into spindle-shaped MIL-88A(Fe): steering charge flow for enhanced visible-light photocatalytic degradation of ibuprofen. Chem Eng J 349:603–612. https://doi.org/10.1016/j.cej.2018.05.121
Hungerford J, Bhattacharyya S, Tumuluri U et al (2018) DMOF-1 as a representative MOF for SO2 adsorption in both humid and dry conditions. J Phys Chem C 122:23493–23500. https://doi.org/10.1021/acs.jpcc.8b06819
Jadhav SA, Garud HB, Patil AH et al (2019) Recent advancements in silica nanoparticles based technologies for removal of dyes from water. Colloids Interface Sci Commun 30:100181. https://doi.org/10.1016/j.colcom.2019.100181
Jiang D, Xu P, Wang H et al (2018) Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord Chem Rev 376:449–466. https://doi.org/10.1016/j.ccr.2018.08.005
Jiang Y, Liu C, Huang A (2019) EDTA-functionalized covalent organic framework for the removal of heavy-metal ions. ACS Appl Mater Interfaces 11:32186–32191. https://doi.org/10.1021/acsami.9b11850
Jones OA, Lester JN, Voulvoulis N (2005) Pharmaceuticals: a threat to drinking water? Trends Biotechnol 23:163–167. https://doi.org/10.1016/j.tibtech.2005.02.001
Joshi JN, Zhu G, Lee JJ et al (2018) Probing metal-organic framework design for adsorptive natural gas purification. Langmuir 34:8443–8450. https://doi.org/10.1021/acs.langmuir.8b00889
Kang YS, Lu Y, Chen K et al (2019) Metal-organic frameworks with catalytic centers: from synthesis to catalytic application. Coord Chem Rev 378:262–280. https://doi.org/10.1016/j.ccr.2018.02.009
Khan NA, Jhung SH (2015) Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: rapid reaction, phase-selectivity, and size reduction. Coord Chem Rev 285:11–23. https://doi.org/10.1016/j.ccr.2014.10.008
Khandan FM, Afzali D, Sargazi G, Gordan M (2018) Novel uranyl-curcumin-MOF photocatalysts with highly performance photocatalytic activity toward the degradation of phenol red from aqueous solution: effective synthesis route, design and a controllable systematic study. J Mater Sci: Mater Electron 29:18600–18613. https://doi.org/10.1007/s10854-018-9978-z
Khasevani SG, Gholami MR (2018) Engineering a highly dispersed core@shell structure for efficient photocatalysis: a case study of ternary novel BiOI@MIL-88A(Fe)@g-C3N4 nanocomposite. Mater Res Bull 106:93–102. https://doi.org/10.1016/j.materresbull.2018.05.024
Khasevani SG, Gholami MR (2019a) Evaluation of the reaction mechanism for photocatalytic degradation of organic pollutants with MIL-88A/BiOI structure under visible light irradiation. Res Chem Intermed 45:1341–1356. https://doi.org/10.1007/s11164-018-3681-9
Khasevani SG, Gholami MR (2019b) Synthesis of BiOI/ZnFe2O4-metal-organic framework and g-C3N4-based nanocomposites for applications in photocatalysis. Ind Eng Chem Res 58:9806–9818. https://doi.org/10.1021/acs.iecr.8b05871
Khasevani SG, Mohaghegh N, Gholami MR (2017) Kinetic study of navy blue photocatalytic degradation over Ag3PO4/BiPO4@MIL-88B(Fe)@g-C3N4 core@shell nanocomposite under visible light irradiation. New J Chem 41:10390–10396. https://doi.org/10.1039/c7nj01968h
Khodkar A, Khezri SM, Pendashteh AR et al (2019) A designed experimental approach for photocatalytic degradation of paraquat using α-Fe2O3@MIL-101(Cr)@TiO2 based on metal-organic framework. Int J Environ Sci Technol 16:5741–5756. https://doi.org/10.1007/s13762-018-1941-2
Kim KC (2018) Design strategies for metal-organic frameworks selectively capturing harmful gases. J Organomet Chem 854:94–105. https://doi.org/10.1016/j.jorganchem.2017.11.017
Kim MK, Kim SH, Park M et al (2018) Degradation of chemical warfare agents over cotton fabric functionalized with UiO-66-NH2. RSC Adv 8:41633–41638. https://doi.org/10.1039/c8ra06805d
Kim S, Gupta NK, Bae J, Kim KS (2020) Structural variations and generation of binding sites in Fe-loaded ZSM-5 and silica under the effect of UV-irradiation and their role in enhanced BTEX abatement from gas streams. J Hazard Mater 384:121274. https://doi.org/10.1016/j.jhazmat.2019.121274
Klimakow M, Klobes P, Thünemann AF et al (2010) Mechanochemical synthesis of metal-organic frameworks: a fast and facile approach toward quantitative yields and high specific surface areas. Chem Mater 22:5216–5221. https://doi.org/10.1021/cm1012119
Kökçam-Demir Ü, Goldman A, Esrafili L et al (2020) Coordinatively unsaturated metal sites (open metal sites) in metal-organic frameworks: design and applications. Chem Soc Rev 49:2751–2798. https://doi.org/10.1039/c9cs00609e
Kumar P, Anand B, Tsang YF et al (2019) Regeneration, degradation, and toxicity effect of MOFs: opportunities and challenges. Environ Res 176:108488. https://doi.org/10.1016/j.envres.2019.05.019
Lee YR, Kim J, Ahn WS (2013) Synthesis of metal-organic frameworks: a mini review. Korean J Chem Eng 30:1667–1680. https://doi.org/10.1007/s11814-013-0140-6
Lee YR, Jang MS, Cho HY et al (2015) ZIF-8: a comparison of synthesis methods. Chem Eng J 271:276–280. https://doi.org/10.1016/j.cej.2015.02.094
Li J, Yang J, Liu YY, Ma JF (2015a) Two heterometallic-organic frameworks composed of iron(III)-salen-based ligands and d10 metals: gas sorption and visible-light photocatalytic degradation of 2-chlorophenol. Chem-A Eur J 21:4413–4421. https://doi.org/10.1002/chem.201406349
Li Y, Wang LJ, Fan HL et al (2015b) Removal of sulfur compounds by a copper-based metal organic framework under ambient conditions. Energy Fuels 29:298–304. https://doi.org/10.1021/ef501918f
Li WJ, Tu M, Cao R, Fischer RA (2016) Metal-organic framework thin films: electrochemical fabrication techniques and corresponding applications & perspectives. J Mater Chem A 4:12356–12369. https://doi.org/10.1039/c6ta02118b
Li H, Li Q, He Y et al (2017) Two novel porous MOFs with square-shaped cavities for the removal of toxic dyes: adsorption or degradation? New J Chem 41:15204–15209. https://doi.org/10.1039/c7nj02904g
Li B, Ma J-G, Cheng P (2018a) Silica-protection-assisted encapsulation of Cu2O nanocubes into a metal-organic framework (ZIF-8) to provide a composite catalyst. Angew Chemie 130:6950–6953. https://doi.org/10.1002/ange.201801588
Li G, Zhao S, Zhang Y, Tang Z (2018b) Metal-organic frameworks encapsulating active nanoparticles as emerging composites for catalysis: recent progress and perspectives. Adv Mater 30:1–43. https://doi.org/10.1002/adma.201800702
Li R, Chen Z, Cai M et al (2018c) Improvement of sulfamethazine photodegradation by Fe(III) assisted MIL-53(Fe)/percarbonate system. Appl Surf Sci 457:726–734. https://doi.org/10.1016/j.apsusc.2018.06.294
Li Y, Jiang J, Fang Y et al (2018d) TiO2 nanoparticles anchored onto the metal-organic framework NH2-MIL-88B(Fe) as an adsorptive photocatalyst with enhanced fenton-like degradation of organic pollutants under visible light irradiation. ACS Sustain Chem Eng 6:16186–16197. https://doi.org/10.1021/acssuschemeng.8b02968
Li H, Zhang J, Yao Y et al (2019a) Nanoporous bimetallic metal-organic framework (FeCo-BDC) as a novel catalyst for efficient removal of organic contaminants. Environ Pollut 255:113337. https://doi.org/10.1016/j.envpol.2019.113337
Li J, Han X, Zhang X et al (2019b) Capture of nitrogen dioxide and conversion to nitric acid in a porous metal-organic framework. Nat Chem 11:1085–1090. https://doi.org/10.1038/s41557-019-0356-0
Li Y, Fang Y, Cao Z et al (2019c) Construction of g-C3N4/PDI@MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl Catal B Environ 250:150–162. https://doi.org/10.1016/j.apcatb.2019.03.024
Li P, Kim S, Jin J et al (2020) Efficient photodegradation of volatile organic compounds by iron-based metal-organic frameworks with high adsorption capacity. Appl Catal B Environ 263:118284. https://doi.org/10.1016/j.apcatb.2019.118284
Liang R, Luo S, Jing F et al (2015) A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl Catal B Environ 176–177:240–248. https://doi.org/10.1016/j.apcatb.2015.04.009
Liang H, Yao A, Jiao X et al (2018a) Fast and sustained degradation of chemical warfare agent simulants using flexible self-supported metal-organic framework filters. ACS Appl Mater Interfaces 10:20396–20403. https://doi.org/10.1021/acsami.8b02886
Liang Q, Jin J, Liu C et al (2018b) Fabrication of the ternary heterojunction Cd0.5Zn0.5S@UIO-66@g-C3N4 for enhanced visible-light photocatalytic hydrogen evolution and degradation of organic pollutants. Inorg Chem Front 5:335–343. https://doi.org/10.1039/c7qi00638a
Liu SQ, Cheng S, Luo L et al (2011) Degradation of dye rhodamine B under visible irradiation with Prussian blue as a photo-Fenton reagent. Environ Chem Lett 9:31–35. https://doi.org/10.1007/s10311-009-0242-x
Liu Y, Howarth AJ, Hupp JT, Farha OK (2015) Selective photooxidation of a mustard-gas simulant catalyzed by a porphyrinic metal-organic framework. Angew Chemie-Int Ed 54:9001–9005. https://doi.org/10.1002/anie.201503741
Liu J, Wei Y, Li P et al (2017a) Selective H2S/CO2 separation by metal-organic frameworks based on chemical-physical adsorption. J Phys Chem C 121:13249–13255. https://doi.org/10.1021/acs.jpcc.7b04465
Liu J, Zhu DD, Guo CX et al (2017b) Design strategies toward advanced MOF-derived electrocatalysts for energy-conversion reactions. Adv Energy Mater 7:1–26. https://doi.org/10.1002/aenm.201700518
Liu B, Vellingiri K, Jo SH et al (2018a) Recent advances in controlled modification of the size and morphology of metal-organic frameworks. Nano Res 11:4441–4467. https://doi.org/10.1007/s12274-018-2039-3
Liu CX, Zhang WH, Wang N et al (2018b) Highly efficient photocatalytic degradation of dyes by a copper–triazolate metal-organic framework. Chem-A Eur J 24:16804–16813. https://doi.org/10.1002/chem.201803306
Liu N, Jing C, Li Z et al (2019) Effect of synthesis conditions on the photocatalytic degradation of rhodamine B of MIL-53(Fe). Mater Lett 237:92–95. https://doi.org/10.1016/j.matlet.2018.11.079
Llabrés i Xamena FX, Corma A, Garcia H (2007) Applications for metal-organic frameworks (MOFs) as quantum dot semiconductors. J Phys Chem C 111:80–85. https://doi.org/10.1021/jp063600e
Lou Z, Xiao X, Huang M et al (2019) Acrylic acid-functionalized metal-organic frameworks for Sc(III) selective adsorption. ACS Appl Mater Interfaces 11:11772–11781. https://doi.org/10.1021/acsami.9b00476
Ma X, Liu H, Li W et al (2016) Reactive adsorption of low concentration methyl mercaptan on a Cu-based MOF with controllable size and shape. RSC Adv 6:96997–97003. https://doi.org/10.1039/c6ra18593b
Ma X, Peng S, Li W et al (2018) Efficient removal of low concentration methyl mercaptan by HKUST-1 membrane constructed on porous alumina granules. Cryst Eng Comm 20:407–411. https://doi.org/10.1039/c7ce01922j
Mahmoodi NM, Abdi J, Oveisi M et al (2018) Metal-organic framework (MIL-100 (Fe)): synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater Res Bull 100:357–366. https://doi.org/10.1016/j.materresbull.2017.12.033
Mahour R, Khan MF, Forbes S, Perez-Estrada LA (2014) Pesticides and herbicides. Water Environ Res 86:1545–1578. https://doi.org/10.2175/106143014x14031280668777
Malik A, Nath M (2019) Multicore-shell nanocomposite formed by encapsulation of WO3 in zeolitic imidazolate framework (ZIF-8): as an efficient photocatalyst. J Environ Chem Eng 7:103401. https://doi.org/10.1016/j.jece.2019.103401
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87:105–145. https://doi.org/10.1016/j.apcatb.2008.09.017
Martínková L, Kotik M, Marková E, Homolka L (2016) Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: a review. Chemosphere 149:373–382. https://doi.org/10.1016/j.chemosphere.2016.01.022
Masoomi MY, Bagheri M, Morsali A, Junk PC (2016) High photodegradation efficiency of phenol by mixed-metal-organic frameworks. Inorg Chem Front 3:944–951. https://doi.org/10.1039/c6qi00067c
Mateo D, Santiago-Portillo A, Albero J et al (2019) Long-term photostability in terephthalate metal-organic frameworks. Angew Chemie-Int Ed 58:17843–17848. https://doi.org/10.1002/anie.201911600
Matthies M, Solomon K, Vighi M et al (2016) The origin and evolution of assessment criteria for persistent, bioaccumulative and toxic (PBT) chemicals and persistent organic pollutants (POPs). Environ Sci Processes Impacts 18:1114–1128. https://doi.org/10.1039/C6EM00311G
McGrath DT, Ryan MD, Macinnis JJ et al (2019) Selective decontamination of the reactive air pollutant nitrous acid: via node-linker cooperativity in a metal-organic framework. Chem Sci 10:5576–5581. https://doi.org/10.1039/c9sc01357a
Mehta JP, Tian T, Zeng Z et al (2018) Sol-gel synthesis of robust metal-organic frameworks for nanoparticle encapsulation. Adv Funct Mater 28:1705588. https://doi.org/10.1002/adfm.201705588
Mei W, Song H, Tian Z et al (2019) Efficient photo-Fenton like activity in modified MIL-53(Fe) for removal of pesticides: regulation of photogenerated electron migration. Mater Res Bull 119:110570. https://doi.org/10.1016/j.materresbull.2019.110570
Meng A, Chaihu L, Chen H et al (2017) Ultrahigh adsorption and singlet-oxygen mediated degradation for efficient synergetic removal of bisphenol A by a stable zirconium-porphyrin metal-organic framework. Sci Rep 7:6297. https://doi.org/10.1038/s41598-017-06194-z
Mohammadi S, Kargari A, Sanaeepur H et al (2015) Phenol removal from industrial wastewaters: a short review. Desalin Water Treat 53:2215–2234. https://doi.org/10.1080/19443994.2014.883327
Momeni MR, Cramer CJ (2018) Dual role of water in heterogeneous catalytic hydrolysis of sarin by zirconium-based metal-organic frameworks. ACS Appl Mater Interfaces 10:18435–18439. https://doi.org/10.1021/acsami.8b03544
Morris W, Doonan CJ, Yaghi OM (2011) Postsynthetic modification of a metal-organic framework for stabilization of a hemiaminal and ammonia uptake. Inorg Chem 50:6853–6855. https://doi.org/10.1021/ic200744y
Naimi Joubani M, Zanjanchi MA, Sohrabnezhad S (2020) The carboxylate magnetic-zinc based metal-organic framework heterojunction: fe3O4-COOH@ZIF-8/Ag/Ag3PO4 for plasmon enhanced visible light Z-scheme photocatalysis. Adv Powder Technol 31:29–39. https://doi.org/10.1016/j.apt.2019.09.034
Nguyen HP, Matsuoka M, Kim TH, Lee SW (2018) Iron(III)-based metal-organic frameworks as potential visible light-driven catalysts for the removal of NOx: a solution for urban air purification. J Photochem Photobiol A Chem 367:429–437. https://doi.org/10.1016/j.jphotochem.2018.09.008
Notar Francesco I, Fontaine-Vive F, Antoniotti S (2014) Synergy in the catalytic activity of bimetallic nanoparticles and new synthetic methods for the preparation of fine chemicals. ChemCatChem 6:2784–2791. https://doi.org/10.1002/cctc.201402252
Oladipo AA (2018) MIL-53 (Fe)-based photo-sensitive composite for degradation of organochlorinated herbicide and enhanced reduction of Cr(VI). Process Saf Environ Prot 116:413–423. https://doi.org/10.1016/j.psep.2018.03.011
Oladipo AA, Vaziri R, Abureesh MA (2018) Highly robust AgIO3/MIL-53 (Fe) nanohybrid composites for degradation of organophosphorus pesticides in single and binary systems: application of artificial neural networks modelling. J Taiwan Inst Chem Eng 83:133–142. https://doi.org/10.1016/j.jtice.2017.12.013
Oladipo AA, Gazi M, Ifebajo AO, et al (2020) Photocatalytic degradation of toxic pesticides. In: Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment. Wiley, pp 93–138. https://doi.org/10.1002/9781119631422.ch4
Patel M, Kumar R, Kishor K et al (2019) Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem Rev 119:3510–3673. https://doi.org/10.1021/acs.chemrev.8b00299
Peterson GW, Mahle JJ, Decoste JB et al (2016) Extraordinary NO2 removal by the metal-organic framework UiO-66-NH2. Angew Chemie-Int Ed 55:6235–6238. https://doi.org/10.1002/anie.201601782
Pirsaheb M, Moradi N (2020) Sonochemical degradation of pesticides in aqueous solution: investigation on the influence of operating parameters and degradation pathway-a systematic review. RSC Adv 10:7396–7423. https://doi.org/10.1039/c9ra11025a
Ploskonka AM, DeCoste JB (2019) Insight into organophosphate chemical warfare agent simulant hydrolysis in metal-organic frameworks. J Hazard Mater 375:191–197. https://doi.org/10.1016/j.jhazmat.2019.04.044
Pratim Bag P, Sahoo P (2020) Designing metal-organic frameworks based photocatalyst for specific photocatalytic reactions: a crystal engineering approach. In: Green photocatalysts for energy and environmental process, pp 142–176. https://doi.org/10.1007/978-3-030-17638-9_6
Prosser RS, Sibley PK (2015) Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ Int 75:223–233. https://doi.org/10.1016/j.envint.2014.11.020
Racles C, Zaltariov MF, Silion M et al (2019) Photo-oxidative degradation of doxorubicin with siloxane MOFs by exposure to daylight. Environ Sci Pollut Res 26:19684–19696. https://doi.org/10.1007/s11356-019-05288-7
Ramezanalizadeh H, Manteghi F (2018) Synthesis of a novel MOF/CuWO4 heterostructure for efficient photocatalytic degradation and removal of water pollutants. J Clean Prod 172:2655–2666. https://doi.org/10.1016/j.jclepro.2017.11.145
Ranft A, Betzler SB, Haase F, Lotsch BV (2013) Additive-mediated size control of MOF nanoparticles. CrystEngComm 15:9296–9300. https://doi.org/10.1039/c3ce41152d
Rawtani D, Khatri N, Tyagi S, Pandey G (2018) Nanotechnology-based recent approaches for sensing and remediation of pesticides. J Environ Manage 206:749–762. https://doi.org/10.1016/j.jenvman.2017.11.037
Rayaroth MP, Aravind UK, Aravindakumar CT (2016) Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ Chem Lett 14:259–290. https://doi.org/10.1007/s10311-016-0568-0
Reinsch H (2016) “Green” synthesis of metal-organic frameworks. Eur J Inorg Chem 2016:4290–4299. https://doi.org/10.1002/ejic.201600286
Rezaei F, Rownaghi AA, Monjezi S et al (2015) SOx/NOx removal from flue gas streams by solid adsorbents: a review of current challenges and future directions. Energy Fuels 29:5467–5486. https://doi.org/10.1021/acs.energyfuels.5b01286
Rieth AJ, Dinca M (2018) Controlled gas uptake in metal-organic frameworks with record ammonia sorption. J Am Chem Soc 140:3461–3466. https://doi.org/10.1021/jacs.8b00313
Rieth AJ, Tulchinsky Y, Dincã M (2016) High and reversible ammonia uptake in mesoporous azolate metal-organic frameworks with open Mn Co, and Ni sites. J Am Chem Soc 138:9401–9404. https://doi.org/10.1021/jacs.6b05723
Rojas S, Horcajada P (2020) Metal-organic frameworks for the removal of emerging organic contaminants in water. Chem Rev 120:8378–8415. https://doi.org/10.1021/acs.chemrev.9b00797
Rubio-Martinez M, Avci-Camur C, Thornton AW et al (2017) New synthetic routes towards MOF production at scale. Chem Soc Rev 46:3453–3480. https://doi.org/10.1039/c7cs00109f
Ruffley JP, Goodenough I, Luo TY et al (2019) Design, Synthesis, and characterization of metal-organic frameworks for enhanced sorption of chemical warfare agent simulants. J Phys Chem C 123:19748–19758. https://doi.org/10.1021/acs.jpcc.9b05574
Safaei M, Foroughi MM, Ebrahimpoor N et al (2019) A review on metal-organic frameworks: synthesis and applications. TrAC-Trends Anal Chem 118:401–425. https://doi.org/10.1016/j.trac.2019.06.007
Sajjadi S, Khataee A, Bagheri N et al (2019) Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J Ind Eng Chem 77:280–290. https://doi.org/10.1016/j.jiec.2019.04.049
Samuel MS, Bhattacharya J, Parthiban C et al (2018) Ultrasound-assisted synthesis of metal organic framework for the photocatalytic reduction of 4-nitrophenol under direct sunlight. Ultrason Sonochem 49:215–221. https://doi.org/10.1016/j.ultsonch.2018.08.004
Sánchez-Sánchez M, Getachew N, Díaz K et al (2015) Synthesis of metal-organic frameworks in water at room temperature: salts as linker sources. Green Chem 17:1500–1509. https://doi.org/10.1039/c4gc01861c
Sarkar S, Banerjee A, Halder U et al (2017) Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv Sci Eng 2:121–131. https://doi.org/10.1007/s41101-017-0031-5
Shah MS, Tsapatsis M, Siepmann JI (2017) Hydrogen sulfide capture: from absorption in polar liquids to oxide, zeolite, and metal–organic framework adsorbents and membranes. Chem Rev 117:9755–9803. https://doi.org/10.1021/acs.chemrev.7b00095
Shen C, Mao Z, Xu H et al (2019) Catalytic MOF-loaded cellulose sponge for rapid degradation of chemical warfare agents simulant. Carbohydr Polym 213:184–191. https://doi.org/10.1016/j.carbpol.2019.02.044
Sheng H, Chen D, Li N et al (2017) Urchin-inspired TiO2@MIL-101 double-shell hollow particles: adsorption and highly efficient photocatalytic degradation of hydrogen sulfide. Chem Mater 29:5612–5616. https://doi.org/10.1021/acs.chemmater.7b01243
Smith GL, Eyley JE, Han X et al (2019) Reversible coordinative binding and separation of sulfur dioxide in a robust metal-organic framework with open copper sites. Nat Mater 18:1358–1365. https://doi.org/10.1038/s41563-019-0495-0
Sokhanvaran V, Gomar M, Yeganegi S (2019) H2S separation from biogas by adsorption on functionalized MIL-47-X (X =–OH and–OCH3): a simulation study. Appl Surf Sci 479:1006–1013. https://doi.org/10.1016/j.apsusc.2019.02.152
Stock N, Biswas S (2012) Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev 112:933–969. https://doi.org/10.1021/cr200304e
Sun Q, Liu M, Li K et al (2017) Synthesis of Fe/M (M = Mn Co, Ni) bimetallic metal organic frameworks and their catalytic activity for phenol degradation under mild conditions. Inorg Chem Front 4:144–153. https://doi.org/10.1039/C6QI00441E
Sun X, Shi Y, Zhang W et al (2018) A new type Ni-MOF catalyst with high stability for selective catalytic reduction of NOx with NH3. Catal Commun 114:104–108. https://doi.org/10.1016/j.catcom.2018.06.012
Sun H, Liu Z, Wang Y et al (2019a) Novel metal-organic framework supported manganese oxides for the selective catalytic reduction of NOx with NH3: promotional role of the support. J Hazard Mater 380:120800. https://doi.org/10.1016/j.jhazmat.2019.120800
Sun H, Zhang H, Mao H et al (2019b) Facile synthesis of the magnetic metal-organic framework Fe3O4/Cu3(BTC)2 for efficient dye removal. Environ Chem Lett 17:1091–1096. https://doi.org/10.1007/s10311-018-00833-1
Tan K, Canepa P, Gong Q et al (2013) Mechanism of preferential adsorption of SO2 into two microporous paddle wheel frameworks M(bdc)(ted)0.5. Chem Mater 25:4653–4662. https://doi.org/10.1021/cm401270b
Tang J, Wang J (2018) Metal organic framework with coordinatively unsaturated sites as efficient fenton-like catalyst for enhanced degradation of sulfamethazine. Environ Sci Technol 52:5367–5377. https://doi.org/10.1021/acs.est.8b00092
Tang J, Wang J (2020) Iron-copper bimetallic metal-organic frameworks for efficient Fenton-like degradation of sulfamethoxazole under mild conditions. Chemosphere 241:125002. https://doi.org/10.1016/j.chemosphere.2019.125002
Tarkwa JB, Oturan N, Acayanka E et al (2019) Photo-Fenton oxidation of Orange G azo dye: process optimization and mineralization mechanism. Environ Chem Lett 17:473–479. https://doi.org/10.1007/s10311-018-0773-0
Tchalala MR, Bhatt PM, Chappanda KN et al (2019) Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat Commun 10:1–10. https://doi.org/10.1038/s41467-019-09157-2
Tian H, Peng J, Du Q et al (2018) One-pot sustainable synthesis of magnetic MIL-100(Fe) with novel Fe3O4 morphology and its application in heterogeneous degradation. Dalt Trans 47:3417–3424. https://doi.org/10.1039/c7dt04819j
Tilgner D, Friedrich M, Verch A et al (2018) A metal-organic framework supported nonprecious metal photocatalyst for visible-light-driven wastewater treatment. Chem Photo Chem 2:349–352. https://doi.org/10.1002/cptc.201700222
Tiwari A, Sagara PS, Varma V, Randhawa JK (2019) Bimetallic metal organic frameworks as magnetically separable heterogeneous catalysts and photocatalytic dye degradation. ChemPlusChem 84:136–141. https://doi.org/10.1002/cplu.201800546
UNESCO (2019) Water and climate change. In: International encyclopedia of geography, pp 1–6
Vaitsis C, Sourkouni G, Argirusis C (2019) Metal organic frameworks (MOFs) and ultrasound: a review. Ultrason Sonochem 52:106–119. https://doi.org/10.1016/j.ultsonch.2018.11.004
Vega F, Baena-Moreno FM, Fernández LMG et al (2020) Current status of CO2 chemical absorption research applied to CCS: towards full deployment at industrial scale. Appl Energy 260:114313. https://doi.org/10.1016/j.apenergy.2019.114313
Vellingiri K, Kumar P, Deep A, Kim K (2016a) Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem Eng J 307:1116–1126. https://doi.org/10.1016/j.cej.2016.09.012
Vellingiri K, Szulejko JE, Kumar P et al (2016b) Metal organic frameworks as sorption media for volatile and semi-volatile organic compounds at ambient conditions. Sci Rep 6:27813. https://doi.org/10.1038/srep27813
Villegas LGC, Mashhadi N, Chen M et al (2016) A short review of techniques for phenol removal from wastewater. Curr Pollut Reports 2:157–167. https://doi.org/10.1007/s40726-016-0035-3
Vu HT, Tran LT, Le GH et al (2019) Synthesis and application of novel Fe-MIL-53/GO nanocomposite for photocatalytic degradation of reactive dye from aqueous solution. Vietnam J Chem 57:681–685. https://doi.org/10.1002/vjch.201900055
Wang SS, Yang GY (2015) Recent advances in polyoxometalate-catalyzed reactions. Chem Rev 115:4893–4962. https://doi.org/10.1021/cr500390v
Wang XL, Fan HL, Tian Z et al (2014) Adsorptive removal of sulfur compounds using IRMOF-3 at ambient temperature. Appl Surf Sci 289:107–113. https://doi.org/10.1016/j.apsusc.2013.10.115
Wang H, Yuan X, Wu Y et al (2015) Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl Catal B Environ 174–175:445–454. https://doi.org/10.1016/j.apcatb.2015.03.037
Wang H, Yuan X, Wu Y et al (2016) In situ synthesis of In2S3 at MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl Catal B Environ 186:19–29. https://doi.org/10.1016/j.apcatb.2015.12.041
Wang Y, Zhang LJ, Zhang R et al (2017) Porous metal-organic frameworks with 5-aminoisophthalic acid as platforms for functional applications about high photodegradation efficiency of phenol. Cryst Growth Des 17:6531–6540. https://doi.org/10.1021/acs.cgd.7b01190
Wang D, Jiang H, Tan J et al (2019a) Manipulating oxidation states of copper within Cu-BTC using Na2S2O3 as a new strategy for enhanced adsorption of sulfide. Ind Eng Chem Res 58:19503–19510. https://doi.org/10.1021/acs.iecr.9b04349
Wang H, Cui PH, Shi JX et al (2019b) Controllable self-assembly of CdS@NH2-MIL-125(Ti) heterostructure with enhanced photodegradation efficiency for organic pollutants through synergistic effect. Mater Sci Semicond Process 97:91–100. https://doi.org/10.1016/j.mssp.2019.03.016
Wang H, Mahle JJ, Tovar TM et al (2019c) Solid-phase detoxification of chemical warfare agents using zirconium-based metal organic frameworks and the moisture effects: analyze via digestion. ACS Appl Mater Interfaces 11:21109–21116. https://doi.org/10.1021/acsami.9b04927
Wang H, Bai JQ, Yin Y, Wang SF (2020) Experimental and numerical study of SO2 removal from a CO2/SO2 gas mixture in a Cu-BTC metal organic framework. J Mol Graph Model 96:107533. https://doi.org/10.1016/j.jmgm.2020.107533
Wei Y, Wang B, Cui X et al (2018) Highly advanced degradation of thiamethoxam by synergistic chemisorption-catalysis strategy using MIL(Fe)/Fe-SPC composites with ultrasonic irradiation. ACS Appl Mater Interfaces 10:35260–35272. https://doi.org/10.1021/acsami.8b12908
Wu XY, Qi HX, Ning JJ et al (2015) One silver(I)/tetraphosphine coordination polymer showing good catalytic performance in the photodegradation of nitroaromatics in aqueous solution. Appl Catal B Environ 168–169:98–104. https://doi.org/10.1016/j.apcatb.2014.12.024
Xue Y, Wang P, Wang C, Ao Y (2018) Efficient degradation of atrazine by BiOBr/UiO-66 composite photocatalyst under visible light irradiation: environmental factors, mechanisms and degradation pathways. Chemosphere 203:497–505. https://doi.org/10.1016/j.chemosphere.2018.04.017
Yang K, Sun Q, Xue F, Lin D (2011) Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: influence of molecular size and shape. J Hazard Mater 195:124–131. https://doi.org/10.1016/j.jhazmat.2011.08.020
Yang F, Liu QK, Ma JP et al (2015) Reversible adsorption and separation of chlorocarbons and BTEX based on Cu(II)-metal organic framework. CrystEngComm 17:4102–4109. https://doi.org/10.1039/c5ce00547g
Yang FW, Li YX, Ren FZ et al (2019a) Toxicity, residue, degradation and detection methods of the insecticide triazophos. Environ Chem Lett 17:1769–1785. https://doi.org/10.1007/s10311-019-00910-z
Yang X, Liang T, Sun J et al (2019b) Template-directed synthesis of photocatalyst-encapsulating metal-organic frameworks with boosted photocatalytic activity. ACS Catal 9:7486–7493. https://doi.org/10.1021/acscatal.9b01783
Yao P, Liu H, Wang D et al (2018) Enhanced visible-light photocatalytic activity to volatile organic compounds degradation and deactivation resistance mechanism of titania confined inside a metal-organic framework. J Colloid Interface Sci 522:174–182. https://doi.org/10.1016/j.jcis.2018.03.075
Yap MH, Fow KL, Chen GZ (2017) Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ 2:218–245. https://doi.org/10.1016/j.gee.2017.05.003
Zeng L, Guo X, He C, Duan C (2016) Metal-organic frameworks: versatile materials for heterogeneous photocatalysis. ACS Catal 6:7935–7947. https://doi.org/10.1021/acscatal.6b02228
Zhang C, Ai L, Jiang J (2015) Solvothermal synthesis of MIL-53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J Mater Chem A 3:3074–3081. https://doi.org/10.1039/c4ta04622f
Zhang Z, Li X, Liu B et al (2016) Hexagonal microspindle of NH2-MIL-101(Fe) metal-organic frameworks with visible-light-induced photocatalytic activity for the degradation of toluene. RSC Adv 6:4289–4295. https://doi.org/10.1039/c5ra23154j
Zhang H, Nai J, Yu L, Lou XW (2017) Metal-organic-framework-based materials as platforms for renewable energy and environmental applications. Joule 1:77–107. https://doi.org/10.1016/j.joule.2017.08.008
Zhang WQ, Cheng K, Zhang H et al (2018) Highly efficient and selective photooxidation of sulfur mustard simulant by a triazolobenzothiadiazole-moiety-functionalized metal-organic framework in air. Inorg Chem 57:4230–4233. https://doi.org/10.1021/acs.inorgchem.8b00106
Zhang H, Chen S, Zhang H et al (2019a) Carbon nanotubes-incorporated MIL-88B-Fe as highly efficient Fenton-like catalyst for degradation of organic pollutants. Front Environ Sci Eng 13:18. https://doi.org/10.1007/s11783-019-1101-z
Zhang R, Du B, Li Q et al (2019b) α-Fe2O3 nanoclusters confined into UiO-66 for efficient visible-light photodegradation performance. Appl Surf Sci 466:956–963. https://doi.org/10.1016/j.apsusc.2018.10.048
Zhang X, Yang Y, Lv X et al (2019c) Adsorption/desorption kinetics and breakthrough of gaseous toluene for modified microporous-mesoporous UiO-66 metal organic framework. J Hazard Mater 366:140–150. https://doi.org/10.1016/j.jhazmat.2018.11.099
Zhang X, Yang Y, Song L et al (2019d) Enhanced adsorption performance of gaseous toluene on defective UiO-66 metal organic framework: equilibrium and kinetic studies. J Hazard Mater 365:597–605. https://doi.org/10.1016/j.jhazmat.2018.11.049
Zhang Y, Chen Z, Liu X et al (2020a) Efficient SO2 removal using a microporous metal-organic framework with molecular sieving effect. Ind Eng Chem Res 59:874–882. https://doi.org/10.1021/acs.iecr.9b06040
Zhang Y, Zhang X, Chen Z et al (2020b) A flexible interpenetrated zirconium-based metal-organic framework with high affinity toward ammonia. Chemsuschem 13:1710–1714. https://doi.org/10.1002/cssc.202000306
Zhao H, Chen Y, Peng Q et al (2017) Catalytic activity of MOF(2Fe/Co)/carbon aerogel for improving H2O2 and ·OH generation in solar photo–electro–Fenton process. Appl Catal B Environ 203:127–137. https://doi.org/10.1016/j.apcatb.2016.09.074
Zhao X, Zhang S, Yan J et al (2018) Polyoxometalate-based metal-organic frameworks as visible-light-induced photocatalysts. Inorg Chem 57:5030–5037. https://doi.org/10.1021/acs.inorgchem.8b00098
Zheng TR, Qian LL, Li M et al (2018) A bifunctional cationic metal-organic framework based on unprecedented nonanuclear copper(II) cluster for high dichromate and chromate trapping and highly efficient photocatalytic degradation of organic dyes under visible light irradiation. Dalt Trans 47:9103–9113. https://doi.org/10.1039/c8dt01685b
Zheng XX, Fang ZP, Dai ZJ et al (2020) Iron-based metal-organic frameworks as platform for H2S selective conversion: structure-dependent desulfurization activity. Inorg Chem 59:4483–4492. https://doi.org/10.1021/acs.inorgchem.9b03648
Zhong X, Lu Y, Luo F et al (2018) A nanocrystalline POM@MOFs catalyst for the degradation of phenol: effective cooperative catalysis by metal nodes and POM guests. Chem-A Eur J 24:3045–3051. https://doi.org/10.1002/chem.201705677
Zhong Z, Li M, Fu J et al (2020) Construction of Cu-bridged Cu2O/MIL(Fe/Cu) catalyst with enhanced interfacial contact for the synergistic photo-Fenton degradation of thiacloprid. Chem Eng J 395:125184. https://doi.org/10.1016/j.cej.2020.125184
Zhu H, Liu D (2019) The synthetic strategies of metal-organic framework membranes, films and 2D MOFs and their applications in devices. J Mater Chem A 7:21004–21035. https://doi.org/10.1039/c9ta05383b
Zhu SY, Yan B (2018) Photofunctional hybrids of TiO2 and titanium metal-organic frameworks for dye degradation and lanthanide ion-tuned multi-color luminescence. New J Chem 42:4394–4401. https://doi.org/10.1039/c7nj04786j
Zhu SR, Liu PF, Wu MK et al (2016) Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light. Dalt Trans 45:17521–17529. https://doi.org/10.1039/c6dt02912d
Zhu M, Hu P, Tong Z et al (2017) Enhanced hydrophobic MIL(Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air. Chem Eng J 313:1122–1131. https://doi.org/10.1016/j.cej.2016.11.008
Acknowledgement
Authors are very grateful for the funds [Project#20200451-001] provided by the “Korea Institute of Civil Engineering and Building Technology” (KICT), Republic of Korea. The authors thank LNCAE (Laboratorio Nacional de Conversión y Almacenamiento de Energía) and Laboratorio Nacional de Ciencia, Tecnología y Gestión Integrada del Agua (LNAGUA) for technical support. Authors would like to thank Grayson Gould, Reliance Canada for language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
López, Y.C., Viltres, H., Gupta, N.K. et al. Transition metal-based metal–organic frameworks for environmental applications: a review. Environ Chem Lett 19, 1295–1334 (2021). https://doi.org/10.1007/s10311-020-01119-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01119-1