Abstract
Cell competition allows winner cells to eliminate less fit loser cells in tissues. In Minute cell competition, cells with a heterozygous mutation in ribosome genes, such as RpS3+/− cells, are eliminated by wild-type cells. How cells are primed as losers is partially understood and it has been proposed that reduced translation underpins the loser status of ribosome mutant, or Minute, cells. Here, using Drosophila, we show that reduced translation does not cause cell competition. Instead, we identify proteotoxic stress as the underlying cause of the loser status for Minute competition and competition induced by mahjong, an unrelated loser gene. RpS3+/− cells exhibit reduced autophagic and proteasomal flux, accumulate protein aggregates and can be rescued from competition by improving their proteostasis. Conversely, inducing proteotoxic stress is sufficient to turn otherwise wild-type cells into losers. Thus, we propose that tissues may preserve their health through a proteostasis-based mechanism of cell competition and cell selection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The following publicly available databases were used in this study: FlyBase (https://flybase.org) and the UniProt D. melanogaster proteome (https://www.uniprot.org/proteomes/UP000000803). Source data are provided with this paper. All other data supporting the findings of this study are available upon reasonable request.
Code availability
The Fiji-based custom-made script can be made available to individuals upon reasonable request while we seek to publish it independent of this study.
References
Morata, G. & Ripoll, P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975).
Baker, N. E. Mechanisms of cell competition emerging from Drosophila studies. Curr. Opin. Cell Biol. 48, 40–46 (2017).
Maruyama, T. & Fujita, Y. Cell competition in mammals—novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol. 48, 106–112 (2017).
Merino, M. M. et al. Elimination of unfit cells maintains tissue health and prolongs lifespan. Cell 160, 461–476 (2015).
Brown, S. et al. Correction of aberrant growth preserves tissue homeostasis. Nature 548, 334–337 (2017).
Liu, N. et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 568, 344–350 (2019).
Vishwakarma, M. & Piddini, E. Outcompeting cancer. Nat. Rev. Cancer 20, 187–198 (2020).
Marygold, S. J. et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8, R216 (2007).
Mills, E. W. & Green, R.Ribosomopathies: there’s strength in numbers.Science 358, eaan2755 (2017).
Baker, N. E. Cell competition. Curr. Biol. 21, R11–R15 (2011).
Ajore, R. et al. Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP53 mutations. EMBO Mol. Med. 9, 498–507 (2017).
Baker, N. E. Emerging mechanisms of cell competition. Nat. Rev. Genet. 21, 683–697 (2020).
Rhiner, C. et al. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell 18, 985–998 (2010).
Meyer, S. N. et al. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236 (2014).
Baillon, L., Germani, F., Rockel, C., Hilchenbach, J. & Basler, K. Xrp1 is a transcription factor required for cell competition-driven elimination of loser cells. Sci. Rep. 8, 17712 (2018).
Kale, A., Li, W., Lee, C.-H. & Baker, N. E. Apoptotic mechanisms during competition of ribosomal protein mutant cells: roles of the initiator caspases Dronc and Dream/Strica. Cell Death Differ. 22, 1300–1312 (2015).
Lee, C.-H. et al. A regulatory response to ribosomal protein mutations controls translation, growth, and cell competition. Dev. Cell 46, 456–469.e4 (2018).
Nagata, R., Nakamura, M., Sanaki, Y. & Igaki, T. Cell competition is driven by autophagy. Dev. Cell 51, 99–112.e4 (2019).
Blanco, J., Cooper, J. C. & Baker, N. E. Roles of C/EBP class bZip proteins in the growth and cell competition of Rp (‘Minute’) mutants in Drosophila. eLife 9, e50535 (2020).
Kucinski, I., Dinan, M., Kolahgar, G. & Piddini, E. Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nat. Commun. 8, 136 (2017).
Kale, A. et al. Ribosomal protein S12e has a distinct function in cell competition. Dev. Cell 44, 42–55.e4 (2018).
Milán, M. Survival of the fittest. Cell competition in the Drosophila wing. EMBO Rep. 3, 724–725 (2002).
Moreno, E. & Basler, K. dMyc transforms cells into super-competitors. Cell 117, 117–129 (2004).
Amoyel, M. & Bach, E. A. Cell competition: how to eliminate your neighbours. Development 141, 988–1000 (2014).
Coelho, C. M. A. Growth and cell survival are unevenly impaired in pixie mutant wing discs. Development 132, 5411–5424 (2005).
Moreno, E., Basler, K. & Morata, G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759 (2002).
Li, W. & Baker, N. E. Engulfment is required for cell competition. Cell 129, 1215–1225 (2007).
Imai, Y. et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila.EMBO J. 27, 2432–2443 (2008).
Mader, S., Lee, H., Pause, A. & Sonenberg, N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 15, 4990–4997 (1995).
Tamori, Y. et al. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 8, e1000422 (2010).
Tamori, Y. & Deng, W.-M. Cell competition and its implications for development and cancer. J. Genet. Genomics 38, 483–495 (2011).
Sykiotis, G. P. & Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76–85 (2008).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Jain, A. et al. p62/Sequestosome-1, autophagy-related gene 8, and autophagy in Drosophila are regulated by nuclear factor erythroid 2-related factor 2 (NRF2), independent of transcription factor TFEB. J. Biol. Chem. 290, 14945–14962 (2015).
Silva-Islas, C. A. & Maldonado, P. D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 134, 92–99 (2018).
Lamb, C. A., Yoshimori, T. & Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 (2013).
Mauvezin, C., Ayala, C., Braden, C. R., Kim, J. & Neufeld, T. P. Assays to monitor autophagy in Drosophila. Methods 68, 134–139 (2014).
Chang, Y.-Y. & Neufeld, T. P. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol. Biol. Cell 20, 2004–2014 (2009).
Piddini, E., Marshall, F., Dubois, L., Hirst, E. & Vincent, J.-P. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development 132, 5479–5489 (2005).
Sancho, M. et al. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev. Cell 26, 19–30 (2013).
Paramore, A. & Frantz, S. Bortezomib. Nat. Rev. Drug Discov. 2, 611–612 (2003).
Pandey, U. B. et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 (2007).
Sung, M.-K., Reitsma, J. M., Sweredoski, M. J., Hess, S. & Deshaies, R. J. Ribosomal proteins produced in excess are degraded by the ubiquitin–proteasome system. Mol. Biol. Cell 27, 2642–2652 (2016).
Kraft, C., Deplazes, A., Sohrmann, M. & Peter, M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat. Cell. Biol. 10, 602–610 (2008).
Wyant, G. A. et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360, 751–758 (2018).
Tye, B. W. et al. Proteotoxicity from aberrant ribosome biogenesis compromises cell fitness. eLife 8, e43002 (2019).
Albert, B. et al. A ribosome assembly stress response regulates transcription to maintain proteome homeostasis. eLife 8, e45002 (2019).
Rubinsztein, D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443, 780–786 (2006).
Nezis, I. P. et al. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J. Cell Biol. 180, 1065–1071 (2008).
Cullinan, S. B. & Diehl, J. A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 279, 20108–20117 (2004).
Bjedov, I. et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46 (2010).
Li, J., Kim, S. G. & Blenis, J. Rapamycin: one drug, many effects. Cell Metab. 19, 373–379 (2014).
Laplante, M. & Sabatini, D. M. mTOR signaling. Cold Spring Harb. Perspect. Biol. 4, a011593 (2012).
Webb, A. E. & Brunet, A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem. Sci. 39, 159–169 (2014).
Klockgether, T., Mariotti, C. & Paulson, H. L. Spinocerebellar ataxia. Nat. Rev. Dis. Prim. 5, 24 (2019).
Bonini, N. M. A genetic model for human polyglutamine-repeat disease in Drosophila melanogaster. Phil. Trans. R. Soc. Lond. B Biol. Sci. 354, 1057–1060 (1999).
Recasens-Alvarez, C. et al. Ribosomopathy-associated mutations cause proteotoxic stress that is alleviated by TOR inhibition. Nat. Cell Biol. (2020).
Coelho, D. S. et al. Culling less fit neurons protects against amyloid-β-induced brain damage and cognitive and motor decline. Cell Rep. 25, 3661–3673.e3 (2018).
Guang, M. H. Z. et al. Targeting proteotoxic stress in cancer: a review of the role that protein quality control pathways play in oncogenesis. Cancers 11, 66 (2019).
Jarosz, D. F., Taipale, M. & Lindquist, S. Protein homeostasis and the phenotypic manifestation of genetic diversity: principles and mechanisms. Annu. Rev. Genet. 44, 189–216 (2010).
Kaushik, S. & Cuervo, A. M. Proteostasis and aging. Nat. Med. 21, 1406–1415 (2015).
Taylor, R. C. & Dillin, A. Aging as an event of proteostasis collapse. Cold Spring Harb. Perspect. Biol. 3, a004440 (2011).
Katheder, N. S. et al. Microenvironmental autophagy promotes tumour growth. Nature 541, 417–420 (2017).
Gay, P. & Contamine, D. Study of the ref(2)P locus of Drosophila melanogaster. II. Genetic studies of the 37DF region. Mol. Gen. Genet. 239, 361–370 (1993).
Zhou, Q., Neal, S. J. & Pignoni, F. Mutant analysis by rescue gene excision: new tools for mosaic studies in Drosophila. Genesis 54, 589–592 (2016).
Acknowledgements
We thank the Piddini group for input on the project and manuscript and R. Carazo Salas for feedback and discussions on the data. We thank the Wolfson Bioimaging Facility for access to microscopes and for assistance in performing the electron microscopy. We thank the University of Bristol Proteomics Facility for performing the TMT proteomics experiments and for proteomics bioinformatics support. We are grateful to T. E. Rusten for the generous gift of the p62 antibody. This work was supported by Wellcome Trust PhD studentships to M.P.D. and I.K., a Cancer Research UK Programme grant to E.P. (A12460), a Cancer Research UK Programme Foundation Award to E.P. (grant C38607/A26831) and a Royal Society University Research Fellowship to E.P. (UF0905080). E.P. is a Wellcome Trust Senior Research Fellow (205010/Z/16/Z).
Author information
Authors and Affiliations
Contributions
E.P. led the project. All authors conceived of the experiments. M.P.D., M.E.B., I.K. and P.F.L. performed and analysed the experiments. M.P.D., M.E.B., P.F.L. and E.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
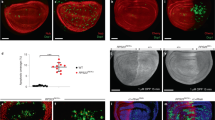
Extended Data Fig. 1 Protein synthesis and its regulation in Rps3+/− cells.
(a-c) AHA (grey) protein synthesis assay in wing discs harboring either Rps3+/− clones (GFP-positive) (a) or clones overexpressing 4EBPTA (GFP-positive) (b), and corresponding quantification (n = 7 and 7, respectively, two-sided paired t-test without p-adjustment for multiple comparisons) (c). (d-e) OPP (green) protein synthesis assay in a wing disc expressing mahj-RNAi in the P compartment (positively labelled with RFP) (d) and corresponding quantification (n = 10, two-sided Wilcoxon signed-rank test) (e). (f-g) An RpS3+/− wing disc expressing GADD34 in the P compartment and labelled with phospho-eIF2α (red) (f), and corresponding quantification (n = 10, two-sided paired t-test) (g). (h-i) GST-GFP reporter (green) activation in an RpS3+/− wing disc expressing GADD34 in the P compartment (h), and corresponding quantification (n = 10, two-sided paired t-test) (i). For all micrographs, scale bars correspond to 50 µm. For all quantifications provided, the horizontal line represents the mean and whiskers indicate 95% confidence intervals. All n numbers refer to the number of individual wing discs.
Extended Data Fig. 2 The role of autophagy in prospective losers.
(a) GstD1-GFP signal (green) in a RpS3+/− wing disc expressing Puc in P cells (labelled by the absence of Ci, magenta). (b-c) Apoptotic cell death, as detected by anti-cleaved Caspase-3 reactivity (red), in wing discs of an atg8+/− heterozygote (b, left), RpS3+/− heterozygote (b, middle), or atg8+/−, RpS3+/− transheterozygote (b, right) and corresponding quantification (n = 9, 8, and 9 respectively, two-sided two sample Kolmgorov-Smirnov test without p-adjustment for multiple comparisons) (c). (d) Apoptotic cell death, as detected by anti-cleaved Caspase-3 reactivity (red), in wing discs of an atg13+/− heterozygote (d, left), RpS3+/− heterozygote (d, middle), or atg13+/−, RpS3+/− transheterozygote (d, right). (e-f) Apoptotic cell death, as detected by anti-cleaved dcp1 antibody staining (red), in wing discs of a p62+/− heterozygote (f, left), RpL27A+/− heterozygote (f, middle), or RpL27A+/−, p62+/− transheterozygote (f, right) and corresponding quantification (n = 10, 10, and 12 respectively, two-sided Mann-Whitney U test without p-adjustment for multiple comparisons) (e). (g-i) Wing discs harboring RpS3+/− clones (GFP-positive) (h, left), RpS3+/− clones expressing atg1-RNAi (GFP-positive) (h, middle), or RpS3+/− clones expressing atg9-RNAi (GFP-positive) (h, right) stained with cleaved-dcp1 (red) and corresponding quantification of border cell death (n = 16, 12, and 9 respectively, two-sided Mann-Whitney U test without p-adjustment for multiple comparisons) (g) and clone coverage (n = 16, 12, and 9 respectively, two-sided student’s t-test without p-adjustment for multiple comparisons) (i). For all micrographs, scale bars correspond to 50 µm. For all quantifications provided, the horizontal line represents the mean and whiskers indicate 95% confidence intervals. All n numbers refer to the number of individual wing discs.
Extended Data Fig. 3 Autophagy flux in ribosome mutants and upon translation inhibition.
(a-c) GFP-p62 ReFlux signal (green) in wing discs expressing RNAi against the autophagy gene atg1 specifically in P cells (labelled by the absence of Ci, magenta), immediately after heat shock (a) or three hours later (b), and corresponding signal quantifications (n = 7 and 6 respectively, two-sided two sample Kolmgorov-Smirnov test) (c). (d-f) GFP-p62 ReFlux signal (green) in a wing disc harboring RpS3+/− clones (dsRed-positive) three hours after heat-shock (d) and corresponding quantification of GFP-p62 signal intensity (e) and number of GFP-p62 foci per area (f) (for both measurements, n = 5, two-sided paired t-test). (g) GFP-p62 ReFlux signal (green) in wing discs harboring RpS3+/− A cells and wild-type P cells, three hours after heat-shock, with or without addition of chloroquine, as indicated. (h) GFP-p62 ReFlux signal (green) in wing discs harboring RpS3+/− A cells (dsRed-positive) and wild-type P cells (dsRed-negative) twenty-four hours after heat-shock. (i-k) GFP-p62 ReFlux signal (green) in wing discs harboring wild-type A cells and 4E-BPTA-expressing P cells (labelled by the absence of Ci, magenta), immediately after heat shock (i) or three hours later (j), and corresponding signal quantifications relative to wing discs containing an RpS3+/− A compartment and wildtype P compartment (images not shown) (n = 9 and 8 for 0 and 3 hour 4E-BPTA, and n = 7 and 8 for 0 and 3 hour RpS3+/−, respectively; two-sided two-sample Kolmgorov-Smirnov test without p-adjustment for multiple comparisons) (k). For all micrographs, scale bars correspond to 50 µm. For all quantifications provided, the horizontal line represents the mean and whiskers indicate 95% confidence intervals. All n numbers refer to the number of individual wing discs.
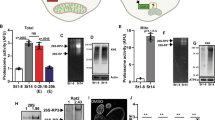
Extended Data Fig. 4 Proteasome defects are linked to the prospective loser status but not to translation inhibition.
(a-b) Apoptosis as detected by anti-cleaved caspase-3 reactivity (green), in Prosβ2+/− (a, left), RpS3+/− (a, middle), or Prosβ2+/−, RpS3+/− transheterozygote (a, right) wing discs and corresponding quantification (n = 10, 10, and 10 respectively, two-sided two sample Kolmgorov-Smirnov test without p-adjustment for multiple comparisons) (b). (c-d) Apoptotic cell death as detected by cleaved-dcp1 (red) in Prosβ2+/− (c, left), a RpL27A+/− (c, middle), or a RpL27A+/−, prosβ2+/− transheterozygote (c, right) wing discs, and corresponding quantification (n = 8, 13, and 10 respectively, two-sided Mann-Whitney U test without p-adjustment for multiple comparisons) (d). (e-g) ProteoFLUX CL1-GFP signal (green) in wing discs expressing mahj-RNAi in the P compartment (RFP-positive), immediately after heat shock (e) or two hours later (f) and corresponding signal quantifications (n = 9 and 7 respectively, two-sided two sample Kolmgorov-Smirnov test) (g). (h-j) ProteoFLUX CL1-GFP signal (green) in wing discs harboring wild-type A cells and 4E-BPTA-expressing P cells (labelled by the absence of Ci, magenta), immediately after heat shock (h) or two hours later (i), and corresponding signal quantifications relative to wing discs containing an RpS3+/− A compartment and wildtype P compartment (images not shown) (n = 9 and 10 for 0 and 2 hour 4E-BPTA, and n = 7 and 7 for 0 and 2 hour RpS3+/−, respectively; two-sided two-sample Kolmgorov-Smirnov test without p-adjustment for multiple comparisons) (j). (k) Transmission Electron microscopy images of a wing disc with wildtype P (left panel) and RpS3+/− A compartments (right panel). Red arrows indicate phago-lysosomal structures containing ribosomes. The scale bar is 500 nm. (l-m) Phospho-eIF2α staining (red) in wing discs harboring RpS3+/− A cells (GFP-positive) and wild-type P cells (GFP-negative) (l) and corresponding signal quantifications (n = 6, two-sided Wilcoxon ranked-sum test) (m). (n-o) A wing disc harboring RpS3+/− clones (GFP-positive) and stained for phospho-eIF2α (red) (n) and corresponding signal quantification (n = 9, two-sided paired t-test) (o). For all micrographs other than those in (k), scale bars correspond to 50 µm. For all quantifications, the horizontal line represents the mean and whiskers indicate 95% confidence intervals. All n numbers refer to the number of individual wing discs.
Extended Data Fig. 5 Proteostasis and the oxidative stress response.
(a-c) GstD1-GFP signal (green) in wild type (a) or RpS3+/− wing discs (b) fed DMSO control or 10μM bortezomib, as indicated, and corresponding quantification (n = 7, 8, 12, and 12, two-sided Mann-Whitney U test without p-adjustment for multiple comparisons) (c). (d-f) Wing discs harboring GFP-positive clones expressing MJDQ27 (d) or MJDQ78 (e) and stained with cleaved-dcp1 (red) and corresponding quantification of cell death (n = 17 and 15 respectively, two-sided Wilcoxon signed-rank test without p-adjustment for multiple comparisons) (f). For all micrographs, scale bars correspond to 50 µm. For all quantifications provided, the horizontal line represents the mean and whiskers indicate 95% confidence intervals. All n numbers refer to the number of individual wing discs.
Supplementary information
Supplementary Tables
Supplementary Tables 1–3
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Baumgartner, M.E., Dinan, M.P., Langton, P.F. et al. Proteotoxic stress is a driver of the loser status and cell competition. Nat Cell Biol 23, 136–146 (2021). https://doi.org/10.1038/s41556-020-00627-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-00627-0
This article is cited by
-
Cell competition and cancer from Drosophila to mammals
Oncogenesis (2024)
-
To not love thy neighbor: mechanisms of cell competition in stem cells and beyond
Cell Death & Differentiation (2023)
-
The PECAn image and statistical analysis pipeline identifies Minute cell competition genes and features
Nature Communications (2023)
-
Cell competition in development, homeostasis and cancer
Nature Reviews Molecular Cell Biology (2023)
-
Nacα protects the larval fat body from cell death by maintaining cellular proteostasis in Drosophila
Nature Communications (2023)