Abstract
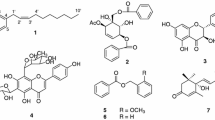
Meteloxetin (1), a new phenolic amino-oxetane, and eight known new source phenols (2–9) have been bioassay-directed isolated from methanolic extract of Datura metel Linn. Their structures were elucidated through modern spectroscopic data. The plant extract showed the significant inhibition potential against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), and its dichloromethane (DCM) fraction exhibited the remarkable inhibition potentials against AChE with IC50 (inhibition concentration) value 1.32 ± 0.02 µg/ml and BChE with IC50 value 1.13 ± 0.01 µg/ml, when compared with the standard drug eserine (AChE, IC50 0.04 ± 0.01 µg/ml) and galanthamine (BChE, IC50 0.92 ± 0.01 µg/ml). The bioactive DCM fraction was subjected to systematic isolation protocol to isolate the 1–9 compounds, and all were subjected to evaluate their AChE and BChE inhibition potentials. From these isolates, compound 1 showed the effective inhibition potential against BChE with IC50 value 0.84 ± 0.03 µg/ml and excellent inhibition potential against AChE with IC50 value 0.07 ± 0.02 µg/ml. This strong inhibition potential of 1 is due to the presence of amino-oxetane groups in it. The in silico studies indicate that oxetane rings contain high-energy oxygen, which makes it a marvelous pharmacophore with diverse biological potentials. The potent nature of compound 1 has also been evaluated by exploring its electronic properties, molecular electrostatic potential and Hirshfeld analysis by density functional theory.





Similar content being viewed by others
References
Ekor, M.: The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharm. 4, 177 (2014)
Newman, D.J.; Cragg, G.M.: Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79(3), 629–661 (2016)
Babalola, S.; Sulaiman, M.; Hassan, A.; Adawa, D.: Evaluation of the crude methanolic seed extract of datura metel l. As a potential oral anaesthetic in dogs. Vet. Res. 6, 115–119 (2013)
Jamdhade, M.; Survase, S.; Kare, M.; Bhuktar, A.: Phytochemical studies on datura metel linn. In marathwada region, maharashtra. J. Phytol. 2(12), 46–48 (2010)
Muthusamy, A.; Punitha, M.; Beslin, L.G.: Phytochemical screening of Datura metel Linn and its antimicrobial activity on selected human pathogens. Int. J. Bioassays 3(11), 3474–3478 (2014)
Sangeetha, S.; Deepa, M.; Sugitha, N.; Mythili, S.; Sathiavelu, A.: Antioxidant activity and phytochemical analysis of Datura metel. Int. J. Drug Dev. Res. 6(4), 46–53 (2014)
Liu, Y.; Guan, W.; Yang, C.-L.; Luo, Y.-M.; Liu, Y.; Zhou, Y.-Y.; Liu, L.-N.; Yang, B.-Y.; Kuang, H.-X.: Steroids with potential anti-inflammatory activity from the roots of datura metel l. Can. J. Chem. 98(2), 74–78 (2020)
Tan, J.-Y.; Liu, Y.; Cheng, Y.-G.; Sun, Y.-P.; Pan, J.; Yang, S.-H.; Kuang, H.-X.; Yang, B.-Y.: Anti-inflammatory sesquiterpenoids from the leaves of Datura metel L. Fitoterapia 142, 104531 (2020)
Yang, B.-Y.; Jiang, H.-B.; Liu, Y.; Chen, J.; Kuang, H.-X.: Steroids from the seeds of datura metel. J. Asian Nat. Prod. Res. 22(3), 257–263 (2018)
Srivastava, N.; Chauhan, A.; Sharma, B.: Isolation and characterization of some phytochemicals from Indian traditional plants. Biotech. Res. Int. 549850, 1–8 (2012)
Srivastava, N.; Sharma, R.; Singh, N.; Sharma, B.: Acetylcholinesterase from human erythrocytes membrane: a screen for evaluating the activity of some traditional plant extracts. Cell. Mol. Biol. 58(1), 160–169 (2012)
Lateef, M.; Azhar, A.; Siddiqui, B.S.; Zarina, S.; Anwar, M.F.; Siddiqui, K.; Azhar, K.F.; Iqbal, L.; Mehmood, R.; Perveen, S.: New anthrarobin acyl derivatives as butyrylcholinesterase inhibitors: synthesis, in vitro and in silico studies. Heliyon 3(7), e00350 (2017)
Lolak, N.; Boga, M.; Tuneg, M.; Karakoc, G.; Akocak, S.; Supuran, C.T.: Sulphonamides incorporating 1, 3, 5-triazine structural motifs show antioxidant, acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibitory profile. J. Enzyme Inhib. Med. Chem. 35(1), 424–431 (2020)
Mehta, M.; Adem, A.; Sabbagh, M.: New acetylcholinesterase inhibitors for alzheimer’s disease. Inter. J. Alzheimer’s Dis (2012). https://doi.org/10.1155/2012/728983
Imran, M.; Irfan, A.; Ibrahim, M.; Assiri, M.A.; Khalid, N.; Ullah, S.; Al-Sehemi, A.G.: Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from oxalis corniculata l. And their first-principles investigations. Ind. Crops Prod. 148, 112285 (2020)
Ibach, B.; Haen, E.: Acetylcholinesterase inhibition in alzheimer’s disease. Current Pharmaceut. Design 10(3), 231–251 (2004)
Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J.: Acetylcholinesterase inhibitors from plants. Phytomedicine 14(4), 289–300 (2007)
Ellman, G.L.; Courtney, K.D.; Andres Jr., V.; Featherstone, R.M.: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 7(2), 88–95 (1961)
Demirtaş, G.; Dege, N.; Ağar, E.; Şahin, S.: The crystallographic, spectroscopic and theoretical studies on (e)-2-[((4-fluorophenyl)imino)methyl]-4-nitrophenol and (e)-2-[((3-fluorophenyl)imino)methyl]-4-nitrophenol compounds. Iran. J. Chem. Chem. Engineer. 37(5), 55–65 (2018)
Irfan, A.; Imran, M.; et al.: Exploration of electronic nature and intrinsic mobility of 10-(1,3-dithiol-2-ylidene)anthracene based organic semiconductor materials. Optik 224, 165530 (2020)
Irfan, A.; Al-Zeidaneen, F.K.; Ahmed, I.; Al-Sehemi, A.G.; Assiri, M.A.; Ullah, S.; Abbas, G.: Synthesis, characterization and quantum chemical study of optoelectronic nature of ferrocene derivatives. Bull. Mater. Sci. 43(1), 45 (2020)
Khalil Warad, I.; Al-Nuri, M.; Ali, O.; Abu-Reidah, I.M.; Barakat, A.; Ben Hadda, T.; Zarrouk, A.; Radi, S.; Touzani, R.; Hicham, E.: Synthesis, physico-chemical, hirschfield surface and dft/b3lyp calculation of two new hexahydropyrimidine heterocyclic compounds. Iran. J. Chem. Chem. Eng. 38(4), 59–68 (2019)
Mikulski, D.; Eder, K.; Molski, M.: Quantum-chemical study on relationship between structure and antioxidant properties of hepatoprotective compounds occurring in cynara scolymus and silybum marianum. J. Theor. Comput. Chem. 13(01), 1450004 (2014)
Najafi, M.; Naqvi, S.A.R.: Theoretical study of the substituent effect on the hydrogen atom transfer mechanism of the irigenin derivatives antioxidant action. J. Theor. Comput. Chem. 13(02), 1450010 (2014)
Sadasivam, K.; Jayaprakasam, R.; Kumaresan, R.: A DFT study on the role of different oh groups in the radical scavenging process. J. Theor. Comput. Chem. 11(04), 871–893 (2012)
Seif, N.; Farhadi, A.; Badri, R.; Kiasat, A.R.: An experimental and theoretical study on bicyclo-3,4-dihydropyrimidinone derivative: synthesis and DFT calculation. Iran. J. Chem. Chem. Eng. (2019). https://doi.org/10.30492/ijcce.2019.35673
Irfan, A.; Assiri, M.; Al-Sehemi, A.G.: Exploring the optoelectronic and charge transfer performance of diaza[5]helicenes at molecular and bulk level. Org. Electron. 57, 211–220 (2018)
Al-Sehemi, A.G.; Irfan, A.: Effect of donor and acceptor groups on radical scavenging activity of phenol by density functional theory. Arab. J. Chem. 10(Supplement 2), S1703–S1710 (2017)
Omura, S.; Murata, M.; Imamura, N.; Iwai, Y.; Tanaka, H.; Furusaki, A.; Matsumoto, T.: Oxetin, a new antimetabolite from an actinomycete. J. Antibiot. 37(11), 1324–1332 (1984)
Bull, J.A.; Croft, R.A.; Davis, O.A.; Doran, R.; Morgan, K.F.: Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem. Rev. 116(19), 12150–12233 (2016)
Akhtar, M.N.; Lam, K.W.; Abas, F.; Ahmad, S.; Shah, S.A.A.; Choudhary, M.I.; Lajis, N.H.: New class of acetylcholinesterase inhibitors from the stem bark of knema laurina and their structural insights. Bioorg. Med. Chem. Lett. 21(13), 4097–4103 (2011)
Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M.: Oxetane-containing metabolites: origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 103(6), 2449–2467 (2019)
Singh, P.K.; Silakari, O.: The current status of o-heterocycles: a synthetic and medicinal overview. Chem. Med. Chem. 13(11), 1071–1087 (2018)
Geerlings, P.; De Proft, F.; Langenaeker, W.: Conceptual density functional theory. Chem. Rev. 103(5), 1793–1874 (2003)
Vektariene, A.; Vektaris, G.; Svoboda, J.: A theoretical approach to the nucleophilic behavior of benzofused thieno [3,2-b] furans using dft and hf based reactivity descriptors. Arkivoc 7, 311–329 (2009)
Acknowledgements
The authors would like to acknowledge the support from the Deanship of Scientific Research at the King Khalid University for funding through the research groups program under Grant Number R.G.P.2/76/41.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Imran, M., Mehmood, R., Hussain, R. et al. Meteloxetin (1) Novel Phenolic Amino-Oxetane Cholinesterase Inhibitors from Datura metel Linn and First-Principle Investigations. Arab J Sci Eng 46, 5681–5690 (2021). https://doi.org/10.1007/s13369-020-05237-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05237-4