Abstract
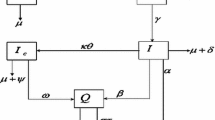
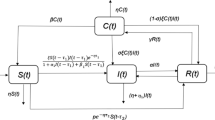
Individuals living with HIV/AIDS are significantly at higher risk of infection with Salmonella Typhi. A deterministic nonlinear mathematical model that describes the co-infection dynamics of HIV and typhoid incorporating typhoid vaccine and treatment has been developed. The basic reproduction number for the co-infection model is computed. The co-infection model exhibits four steady states, namely, the disease-free, HIV alone endemic, typhoid alone endemic, and the co-infection endemic states. The local stability of the disease-free state has been investigated. The co-infection endemic state, if it exists, is found to be locally stable. The minimum vaccination rate that eliminates the typhoid infection is determined. Sensitivity analysis has been performed to ascertain model parameters that have a strong impact on the disease dynamics. It is demonstrated that the co-infection basic reproduction number can be reduced to below unity by simultaneous preventive measures, thereby eliminating both diseases. Numerical simulation of the co-infection model is carried out to examine the effects of parameters on disease spread. The study suggests that the two diseases need to be managed simultaneously for effective control of the co-infection.








Similar content being viewed by others
References
Agwu, E., Ihongbe, J., Okogun, G., Inyang, N.: High incidence of co-infection with malaria and typhoid in febrile hiv infected and aids patients in ekpoma, edo state, nigeria. Braz. J. Microbiol. 40(2), 329–332 (2009)
Angulo, F.J., Swerdlow, D.L.: Bacterial enteric infections in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 21(Supplement–1), S84–S93 (1995)
Antillón, M., Warren, J.L., Crawford, F.W., Weinberger, D.M., Kürüm, E., Pak, G.D., Marks, F., Pitzer, V.E.: The burden of typhoid fever in low-and middle-income countries: A meta-regression approach. PLoS Negl. Tropical Dis. 11(2), e0005376 (2017)
Baker, S., Holt, K.E., Clements, A.C., Karkey, A., Arjyal, A., Boni, M.F., Dongol, S., Hammond, N., Koirala, S., Duy, P.T., et al.: Combined high-resolution genotyping and geospatial analysis reveals modes of endemic urban typhoid fever transmission. Open Biol. 1(2), 110008 (2011)
Brachman, P.S., Abrutyn, E.: Bacterial infections of humans: epidemiology and control. Springer, New York (2009)
Butler, T.: Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin. Microbiol. Infect. 17(7), 959–963 (2011)
Carvalho, A.R., Pinto, C.M.: A coinfection model for hiv and hcv. Biosystems 124, 46–60 (2014)
Castillo-Chavez, C., Blower, S., van den Driessche, P., Kirschner, D., Yakubu, A.A.: Mathematical approaches for emerging and reemerging infectious diseases: an introduction. Springer, New York (2002)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1(2), 361–404 (2004)
Celum, C.L., Chaisson, R.E., Rutherford, G.W., Barnhart, J.L., Echenberg, D.F.: Incidence of salmonellosis in patients with aids. J. Infect. Dis. 156, 998–1002 (1987)
Chitnis, N., Hyman, J.M., Cushing, J.M.: sensitivity analysis of a mathematical model. Bull. Math. Biol. 70(5), 1272 (2008)
Cooper, C.: Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 392(10159), 1789–1858 (2018)
Crump, J.A.: Progress in typhoid fever epidemiology. Clin. Infect. Dis. 68(Supplement–1), S4–S9 (2019)
Van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci 180(1–2), 29–48 (2002)
Edward, S., Nyerere, N.: Modelling typhoid fever with education, vaccination and treatment. Eng. Math. 1(1), 44–52 (2016)
Edward, S., et al.: A deterministic mathematical model for direct and indirect transmission dynamics of typhoid fever. Open Access Libr. J. 4(05), 1 (2017)
Fischl, M.A., Dickinson, G.M., Sinave, C., Pitchenik, A.E., Cleary, T.J.: Salmonella bacteremia as manifestation of acquired immunodeficiency syndrome. Arch. Internal Med. 146(1), 113–115 (1986)
González-Guzmán, J.: An epidemiological model for direct and indirect transmission of typhoid fever. Math. Biosci. 96(1), 33–46 (1989)
Gotuzzo, E., Frisancho, O., Sanchez, J., Liendo, G., Carrillo, C., Black, R.E., Morris, J.G.: Association between the acquired immunodeficiency syndrome and infection with salmonella typhi or salmonella paratyphi in an endemic typhoid area. Arch. Internal Med. 151(2), 381–382 (1991)
Kalra, S., Naithani, N., Mehta, S., Swamy, A.: Current trends in the management of typhoid fever. Med. J. Armed Forc. India 59(2), 130 (2003)
Kaur, N., Ghosh, M., Bhatia, S.: Modelling the role of awareness and screening of infectives in the transmission dynamics of hiv. World J Modelling and Simul. 12(2), 97–111 (2016)
Kgosimore, M., Kelatlhegile, G.: Mathematical analysis of typhoid infection with treatment. J. Math. Sci. Adv. Appl 40, 75–91 (2016)
Kroon, F.P., van Dissel, J.T., Ravensbergen, E., Nibbering, P.H., van Furth, R.: Impaired antibody response after immunization of hiv-infected individuals with the polysaccharide vaccine against salmonella typhi (typhim-vi®). Vaccine 17(23–24), 2941–2945 (1999)
LaSalle, J.P.: The stability of dynamical systems. Siam 21(3), 418–420 (1976)
Ma, S., Xia, Y.: Mathematical understanding of infectious disease dynamics. World Scientific, (2009)
Marchello, C.S., Hong, C.Y., Crump, J.A.: Global typhoid fever incidence: A systematic review and meta-analysis. Clin. Infect. Dis. 68(Supplement–2), S105–S116 (2019)
Mathews, J.H., Fink, K.D., et al.: Numer. Methods MATLAB. Pearson prentice hall Upper Saddle River, NJ (2004)
Mogasale, V., Maskery, B., Ochiai, R.L., Lee, J.S., Mogasale, V.V., Ramani, E., Kim, Y.E., Park, J.K., Wierzba, T.F.: Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Global Health 2(10), e570–e580 (2014)
Mushanyu, J., Nyabadza, F., Muchatibaya, G., Mafuta, P., Nhawu, G.: Assessing the potential impact of limited public health resources on the spread and control of typhoid. J Math. Biol. 77(3), 647–670 (2018)
Mushayabasa, S.: Modeling the impact of optimal screening on typhoid dynamics. Int. J. Dyn. and Control 4(3), 330–338 (2016)
Mushayabasa, S., Bhunu, C.P., Mhlanga, N.A.: Modeling the transmission dynamics of typhoid in malaria endemic settings. Applications & Applied Mathematics 9(1), (2014)
Mutua, J.M., Wang, F.B., Vaidya, N.K.: Modeling malaria and typhoid fever co-infection dynamics. Math. Biosci. 264, 128–144 (2015)
Nthiiri, J., Lawi, G., Akinyi, C., Oganga, D., Muriuki, W., Musyoka, M., Otieno, P., Koech, L.: Mathematical modelling of typhoid fever disease incorporating protection against infection. Journal of Advances in Mathematics and Computer Science pp. 1–10 (2016)
Pitzer, V.E., Bowles, C.C., Baker, S., Kang, G., Balaji, V., Farrar, J.J., Grenfell, B.T.: Predicting the impact of vaccination on the transmission dynamics of typhoid in south asia: a mathematical modeling study. PLoS Negl. Trop. Dis. 8(1), e2642 (2014)
Rizvi, F.: Mathematical modeling of two-dose vaccines. Ph.D. thesis, The Ohio State University (2016)
Saha, S., Samanta, G.: Modelling and optimal control of hiv/aids prevention through prep and limited treatment. Phys. A: Stat. Mech. Appl. 516, 280–307 (2019)
Sperber, S.J., Schleupner, C.J.: Salmonellosis during infection with human immunodeficiency virus. Rev. Infect. Dis. 9(5), 925–934 (1987)
Tian, J.P., Wang, J.: Global stability for cholera epidemic models. Math. Biosci. 232(1), 31–41 (2011)
Tilahun, G.T., Makinde, O.D., Malonza, D.: Modelling and optimal control of typhoid fever disease with cost-effective strategies. Comput. Math. Methods Med. 2017, 1–16 (2017)
Tilahun, G.T., Makinde, O.D., Malonza, D.: Co-dynamics of pneumonia and typhoid fever diseases with cost effective optimal control analysis. Appl. Math. Comput. 316, 438–459 (2018)
UNAIDS: Global hiv & aids statistics-2019 fact sheet (2019)
Acknowledgements
The first author is grateful to the Indian Council for Cultural Relations (ICCR) for financial support during his Ph.D. work. The authors are thankful to the anonymous reviewers for their valuable comments and suggestions, which helped us to improve the quality of our original manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Proof of Theorem 1
Proof
The right hand side of the co-infection model system (2.2) is continuous and satisfies locally Lipschitz condition on the space of continuous functions. Thus, its solution \((S(t), I_H(t), I_T(t), I_{HT}(t), I_A(t), I_{AT}(t), R(t), B(t))\) exists and is unique over [0, T), where \(0<T\le \infty \).
Let us first establish that \(S(t)>0\), \(\forall t\in [0,T)\). Suppose this statement is false. Then \(\exists t_1\in [0,T)\) such that \(S(t_1)=0\), \(\frac{dS(t_1)}{dt}\le 0\) and \(S(t)>0\), \(\forall t\in [0,t_1)\). Then there must be \(I_H(t)>0\), \(I_T(t)>0\) and \(R(t)>0\), \(\forall t\in [0,t_1)\). Suppose not. Then \(\exists t_2\in [0,t_1)\) such that
Then there must have \(I_{HT}(t)>0\), \(I_A(t)>0\) and \(B(t)>0\), \(\forall t\in [0,t_2)\). Suppose this is not true. Then \(\exists t_3\in [0,t_2)\) such that
Our claim is \(I_{AT}(t)>0\), \(\forall t\in [0,t_3)\). If this is not true, \(\exists t_4\in [0,t_3)\) such that \(I_{AT}(t_4)=0\), \(\frac{dI_{AT}(t_4)}{dt}\le 0\) and \(I_{AT}( t)>0\), \(\forall t\in [0,t_4)\). From the sixth equation of system (2.2)
which contradicts \(\frac{dI_{AT}(t_4)}{dt}\le 0\). Therefore, \(I_{AT}(t)>0\), \(\forall t\in [0,t_3)\).
So the fourth, fifth, and eighth equations of system (2.2) give
which contradicts \(\frac{dI_{HT}(t_3)}{dt}\le 0\), \(\frac{dI_A(t_3)}{dt}\le 0\) and \(\frac{dB(t_3)}{dt}\le 0\), respectively (See Eq. (A.2)). Hence, \(I_{HT}(t)>0\), \(I_A(t)>0\) and \(B(t)>0\), \(\forall t\in [0,t_2)\). Similarly, \(I_{AT}>0\), \(\forall t\in [0,t_2)\).
Now from the second, third, and seventh equations of system (2.2)
which is a contradiction to \(\frac{dI_H(t_2)}{dt}\le 0\), \(\frac{dI_T(t_2)}{dt}\le 0\) and \(\frac{dR(t_2)}{dt}\le 0\), respectively (See Eq. (A.1)). Hence, \(I_H(t)>0\), \(I_T(t)>0\) and \(R(t)>0\), \(\forall t\in [0,t_1)\). This implies that \(I_{HT}>0\), \(I_A(t)>0\), \(I_{AT}>0\) and \(B(t)>0\), \(\forall t\in [0,t_1)\). It follows from the first equation of system (2.2)
which contradicts \(\frac{dS(t_1)}{dt}\le 0\). Hence, \(S(t)>0\), \(\forall t\in [0,T)\).
The above step-by-step discussion follows that \(I_H(t)>0\), \(I_T(t)>0\), \(I_{HT}(t)>0\), \(I_A(t)>0\), \(I_{AT}(t)>0\), \(R(t)>0\) and \(B(t)>0\) for all \(t\in [0,T)\). Thus, the theorem is proved. \(\square \)
Proof of Theorem 2
Proof
The dynamics of the total human population is
Accordingly,
Thus,
Thus, the total human population N(t) is bounded. Hence each of its coordinates \((S(t), I_H(t), I_T(t), I_{HT}(t), I_A(t), I_{AT}(t), R(t))\) is bounded.
Further, the dynamics of Salmonella Typhi bacteria in the environment is given by
This gives
Thus,
Hence, B(t) is also bounded.
Therefore, every solution of the model system (2.2) with initial conditions in \(\mathbb {R}_+^{8}\) will enter and remains in region
\(\square \)
Rights and permissions
About this article
Cite this article
Irena, T.K., Gakkhar, S. A dynamical model for HIV-typhoid co-infection with typhoid vaccine. J. Appl. Math. Comput. 67, 641–670 (2021). https://doi.org/10.1007/s12190-020-01485-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12190-020-01485-7