Abstract
Understanding the various structures of gold clusters and the interaction modes between Au clusters and biomolecules is an important issue in material science such as biosensors and catalysts. The binding of small gold clusters (Aun n = 2–5) with neutral and anionic forms of arginine (Arg) amino acid is investigated in this study using density functional theory (DFT) and B3LYP level. The relative stability among different forms of Au clusters including linear, zigzag, planar, and three-dimensional Au clusters was estimated, initially. The calculated findings show that the zigzag structure for Au3 and the planar structure for Au4 and Au5 are the best form. Furthermore, the different modes of interaction were taken into account from thermodynamic view point between the most stable conformers of Arg and Arg− with gold clusters. Finally, the arginine is considered as a weak organic acid to investigate the impact of Au clusters on the gas phase acidity. The acidity of isolated arginine and the acidity of [Aun/Arg] complexes were also compared. Based on the obtained results, upon the complexation with Au clusters at 298 K, for the interaction of Au, Au2, Au3, Au4, and Au5 clusters with arginine, the gas phase acidity (GPA) of arginine alters from 342.12 to 314.17, 303.04, 299.42, 303.41, and 331.66 kcal/mol respectively. These calculated values predict that when a weak organic acid is complexed with Au clusters, it will be altered to super acid. Furthermore, for isolated and complexed species of Arg, pKa values were evaluated in water solvent.




Similar content being viewed by others
References
Pyykkö P (2004) Theoretical chemistry of gold. Angew Chem 43:4412
Alvarez BRA, Flores-Lopez NS, Calderón-Ayala G, Britto Hurtado R, Cortez-Valadez M, Flores-Acosta M (2019) First-principles calculations of gold and silver clusters doped with lithium atoms. J. Physica E Low Dimens Syst Nanostruct 109:78–83
Yang Y, Chen S (2003) Surface manipulation of the electronic energy of sub nanometer-sized gold clusters: an electrochemical and spectroscopic investigation. Nano Lett 3:75–79
Rajesh C, Majumder C (2019) Interaction of gold clusters with graphene and graphene layer over surface: a density functional study. J Appl Surf Sci 469:917–922
Lei Z, Wang Q (2019) Homo and heterometallic gold(I) clusters with hypercoordinated carbon. J Coord Chem Re 378:382–394
Pyykkö P (2005) Theoretical chemistry of gold II. Inorg Chem 358:4113
Kurashige W, Kumazawa R, Yoshino S, Negishi Y (2018) Thiolate-protected gold clusters as functional materials in photocatalysts. Encyclopedia of Interfacial Chemistry 683–696
Camacho-Mendoza RL, Zárate-Hernández LA, Vásquez-Pérez JM, Cruz-Borbolla J, Alvarado-Rodríguez JG, Thangarasu P (2018) On the interaction of anisole and thioanisole derivatives with gold clusters studied by DFT. J. Comput Theor Chem 1126:54–64
Sanchez A, Abbet S, Heiz U, Schneider WD, Hakkinen H, Barnett RN, Landman U (1999) When gold is not noble: nanoscale gold catalysts. J Phys Chem A 103:9573–9578
Hakkinen H, Landman U (2001) Gas-phase catalytic oxidation of CO by Au2−. J Am Chem Soc 123:9704–9705
Jabbarzadeh SaniAli M, Pakiari H (2018) Relativistic and nonrelativistic structures, stabilities and electronic properties of small neutral gold clusters. J Comput Theor Chem 1136–1137:18–28
Bond GC, Louis C, Thompson DT (2006) Catalysis by gold. Imperical college Press, London, 6
Schwerdtfeger P, Lien M (2009) Theoretical Chemistry of Gold– From Atoms to Molecules, Clusters, Surfaces and the Solid State. In: Mohr F (ed) Gold chemistry: current trends and future directions in the life sciences, 1st edn. Wiley, New York, p 183
Pyykkö P (1997) Strong closed-Shell interactions in inorganic chemistry. Chem Rev (Washington, DC) 97:597
Morris SM Jr (2007) Arginine metabolism: boundaries of our knowledge. Nutr 137:1602S–1609S
Javan MJ, Jamshidi Z, Tehrania ZA, Fattahi A (2012) Interactions of coinage metal clusters with histidine and their effects on histidine acidity; theoretical investigation. J Org Biomol Chem 10:9373–9382
Morris SM Jr (2012) Arginases and arginine deficiency syndromes. Curr Opin Clin Nutr Metab Care 15:64–70
Vissers YL, Dejong CH, Luiking YC, Fearon KC, von Meyenfeldt MF, Deutz NE (2005) Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am J Clin Nutr 81:1142–1146
He L, So VLL, Xin JH (2014) A new rhodamine-thiourea/Al3+ complex sensor for the fast visual detection of arginine in aqueous media. Sensors Actuators B Chem 192:496–502
Ting L, Li N, Jiang XD, Ying Z, Yu ZF, Shu ML, Hong QL, Nian BL (2017) A colorimetric and fluorometric dual-signal sensor for arginine detection by inhibiting the growth of gold nanoparticles/carbon quantum dots composite. Biosens Bioelectron 87:772–778
Gopalakrishnan V, Burton PJ, Blaschke TF (1996) High-performance liquid chromatographic assay for the quantitation of l-arginine in human plasma. Anal Chem 68:3520–3523
Williams J, Lang D, Smith JA, Lewis MJ (1993) Plasma l-arginine levels in a rabbit model of hypercholesterolaemia. Biochem Pharmacol 46:2097–2099
Olson DL, Lacey ME, Webb AG, Sweedler JV (1999) Sweedler, nanoliter-volume NMR detection using periodic stopped-flow capillary electrophoresis. Anal Chem 71:3070–3076
Wang W, Rusin O, Xu X, Kim KK, Escobedo JO, Fakayode SO, Fletcher KA, Lowry M, Schowalter CM, Lawrence CM, Fronczek FR, Warner IM, Strongin RM (2005) Detection of homocysteine and cysteine. Am Chem Soc 127:15949–15958
Alivisatos AP, Johnsson KP, Peng X, Wilson TE, Loweth CJ, Bruchez MP, Schultz PG (1996) Organization of 'nanocrystal molecules' using DNA. Nat 382:609–611
Parak WJ, Gerion D, Pellegrino T, Zanchet D, Micheel C, Williams SC, Boudreau R, Gros MAL, Larabell CA, Alivisatos AP (2003) Biological applications of colloidal nanocrystals. Nanotechnol 14:829–938
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Sci 277:1078–1080
Cao YWC, Jin RC, Mirkin CA (2002) Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Sci 297:1536–1540
Park HY, Schadt MJ, Wang LY, Lim IIS, Njoki PN, Kim SH, Jang MY, Luo J, Zhong CJ (2007) Fabrication of magnetic core@shell Fe oxide@Au nanoparticles for interfacial bioactivity and bio-separation. Langmuir 23:9050–9056
Ni J, Lipert RJ, Dawson GB, Porte MD (1999) Immunoassay readout method using extrinsic Raman labels adsorbed on immunogold colloids. Anal Chem 71:4903–4908
Grubisha DS, Lipert RJ, Park HY, Driskell J, Porter MD (2003) Femtomolar detection of prostate-specific antigen: an immunoassay based on surface-enhanced Raman scattering and immunogold labels. Anal Chem 75:5936–5943
Ghosh P, Han G, Erdogan B, Rosado O, Krovi SA, Rotello VM (2007) Nanoparticles featuring amino acid-functionalized side chains as DNA receptors. Chem Biol Drug Des 70:13–18
Zhong Z, Patskovskyy S, Bouvrette P, Luong JHT, Gedanken A (2004) The surface chemistry of Au colloids and their interactions with functional amino acids. J Phys Chem B 108:4046–4052
Pakiari AH, Jamshidi Z (2007) Interaction of amino acids with gold and silver clusters. J Phys Chem A 111:4391–4396
Pyykkö P (2007) Structural properties: magic nanoclusters of gold. Nat Nanotechnol 2:273–274
Knickelbein MB (1999) Reactions of transition metal clusters with small molecules. Annu Rev Physiol 50:79–115
Kshirsagar A, Ghebriel HW (2007) Adsorption of molecular hydrogen and hydrogen sulfide on Au clusters. J Chem Phys 126:244705–244714 (c) Fuchs H, Krüger D, Rousseau R, Marx D, Parrinello M (2001) Interaction of short-chain alkane thiols and thiolates with small gold clusters: adsorption structures and energetic. J Chem Phys 115:4776–4786
Lim IIS, Ip W, Crew E, Njoki PN, Mott D, Zhong CJ, Pan Y, Zhou S (2007) Homocysteine-mediated reactivity and assembly of gold nanoparticles. Langmuir 23:826–833
Carey FA, Sundberg RJ (2000) Advanced organic chemistry, 4th edn. Kluwer Academic/Plenum Publishers, New York
Anslyn EV, Dougherty DA (2006) Modern physical organic chemistry. University Science Books, Sausalito
Bell RP (1973) Proton in Chemistry. Chapman and Hall, London
Albert A, Serjeant EP (1984) The determination of ionization constants. Chapman and Hall, New York
Pliego JR Jr, Riveros JM (2002) Theoretical calculation of pKa using the cluster−continuum model. J Phys Chem A 106:7434–7439
Magill AM, Cavell KJ, Yates BF (2004) Basicity of nucleophilic carbenes in aqueous and nonaqueous solvents-theoretical predictions. J Am Chem Soc 126:8717–8724
Jorgensen WL, Briggs JM, Gao J (1987) A priori calculations of pKa's for organic compounds in water. The pKa of Ethane. J Am Chem Soc 109:6857–6858
Becke AD (1993) Density-functional thermochemistry. III The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin JA, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision B.4, Gaussian, Inc., Pittsburgh PA
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys Chem 82:270
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys Chem 82:284–283
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys Chem 82:299–310
Koelling DD, Harmon BN (1977) A technique for relativistic spin-polarised calculations. J Phys C Solid State Phys 10:3107–3114
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Ding F, Smith MJ, Wang H (2009) First-principles calculation of pKa values for organic acids in nonaqueous solution. J Organomet Chem 74:2679–2691
Wang L, Ge Q (2002) Studies of rhodium nanoparticles using the first principles density functional theory calculations. J Chem Phys Lett:366–368
Zhang W, Ge Q, Wang L (2003) Structure effects on the energetic, electronic, and magnetic properties of palladium nanoparticles. J Chem Phys 118:5793–5801
Zhang W, Zhao H, Wang L (2004) The simple cubic structure of ruthenium clusters. J Phys Chem B 108:2140–2147
Zhang W, Xiao L, Hirata Y, Pawluk T, Wang L (2004) The simple cubic structure of Ir clusters and the element effect on cluster structures. J Chem Phys Lett 383:67–71
Pawluk T, Hirata Y, Wang L (2005) Studies of iridium nanoparticles using density functional theory calculations. J Phys Chem B 109:20817–20823
Xiao L, Wang L (2004) Structures of platinum clusters: planar or spherical? J Phys Chem A 108:8605–8614
Ge Q, Song C, Wang L (2006) A density functional theory study of CO adsorption on Pt-Au nanoparticles. Comput Mater Sci 35:247–253
Song C, Ge Q, Wang L (2005) DFT studies of Pt/Au bimetallic clusters and their interactions with the CO molecule. J Phys Chem B 109:22341–22350
Zhang W, Ran X, Zhao H, Wang L (2004) The nonmetallicity of molybdenum clusters. J Chem Phys 121:7717
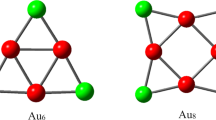
Xiao L, Tollberg B, Hu X, Wang L (2006) Structural study of gold clusters. J Chem Phys 124:114309
Simard B, Hackett PA (1990) High resolution study of the (0, 0) and (1, 1) bands of the A0u+-X0g+. J Mol Spectrosc 142:310–318
Walker AV (2005) Structure and energetics of small gold nanoclusters and their positive ions. J Chem Phys 122:094310
Howard JA, Sutcliffe R, Mile B (1983) E.s.r. spectrum of matrix isolated Au3. J Chem Soc Chem Commun 1449–1450
Gronbeck H, Andreoni W (2000) Gold and platinum microclusters and their anions: comparison of structural and electronic properties. Chem Phys 262:1–14
Bonacic-Koutecky V, Burda J, Mitric R, Ge M, Zampella G, Fantucci P (2002) Density functional study of structural and electronic properties of bimetallic silver–gold clusters: comparison with pure gold and silver clusters. J Chem Phys 117:3120–3131
Wang J, Wang G, Zhao J (2002) Density-functional study of Aun(n=2–20) clusters: lowest-energy structures and electronic properties. Phys Rev B 66:035418
Jamshidi Z, Farhangian H, Tehrani ZA (2012) Glucose interaction with Au, Ag, and Cu clusters: theoretical investigation. Int J Quantum Chem. https://doi.org/10.1002/qua.24122
Tehrani ZA, Jamshidi Z, Javan MJ, Fattahi A (2012) Interactions of glutathione tripeptide with gold cluster: influence of intramolecular hydrogen bond on complexation behavior. J Phys Chem A 116:4338–4347
Xie HJ, Lei QF, Fang WJ (2012) Intermolecular interactions between gold clusters and selected amino acids cysteine and glycine: a DFT study. J Mol Model 2:645–652
Rai S, Suresh kumar NV, Singh H (2012) A theoretical study on interaction of proline with gold cluster. Bull Mater Sci 35:291–295
Kryachko ES, Remacle F (2005) Complexes of DNA bases and gold clusters Au3 and Au4 involving nonconventional N-H...Au hydrogen bonding. Nano Lett 5:735–739
Kumar A, Mishra PC, Suhai S (2006) Binding of gold clusters with DNA base pairs: a density functional study of neutral and anionic GC-Aun and AT-Aun (n = 4, 8) complexes. J Phys Chem A 110:7719–7727
Kryachko ES, Remacle F (2005) Complexes of DNA bases and Watson-crick base pairs with small neutral gold clusters. J Phys Chem B 109:22746–22757
Wang XB, Nicholas JB, Wang LSJ (2000) Photoelectron spectroscopy and theoretical calculations of SO4− and HSO4−: confirmation of high electron affinities of SO4 and HSO4. J Phys Chem A 104:504–508
Kraychko ES (2008) Where gold meets a hydrogen bond? J Mol Struct 880:23–30
Acknowledgments
The authors gratefully acknowledge financial support from the research council of Alzahra University. Technical support of the Chemistry Computation Center at Shahid Beheshti University is greatly acknowledged.
Funding
This study received financial support from the research council of Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1572 kb)
Rights and permissions
About this article
Cite this article
Ghiasi, M., Bavafa, S. & Zahedi, M. QM study of interaction between arginine amino acid and Au clusters and the effects on arginine acidity. Gold Bull 54, 45–57 (2021). https://doi.org/10.1007/s13404-021-00292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-021-00292-7