Abstract
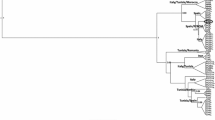
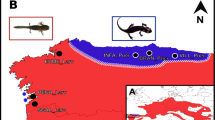
Contact zones present unique opportunities to investigate ecological divergence, reproductive barriers, and gene flow between species. The two-lined salamander (Eurycea bislineata) species complex is a group of semiaquatic plethodontid salamanders with a reticulate evolutionary history that reflects the reorganization of river drainage basins. Although evidence for widespread, ancient introgression suggests an absence of reproductive isolating mechanisms in the early evolutionary history of the group, modern contact zones reveal a broader diversity of outcomes—with some putative species pairs occurring in sympatry and others exhibiting narrow hybrid zones. Here, we used RADcap data to investigate gene flow and ecological divergence in replicate contact zones between two species in the Appalachian foothills. Our results demonstrate that gene flow between these species is absent or rare, and larvae show strong, fine-scale ecological segregation among riffles, runs, and pools in streams. These results reinforce the more ambiguous conclusions of previous studies that suggested the evolutionary distinctiveness of these two species and underscore the importance of ecological factors in shaping local distributions.



Similar content being viewed by others
Availability of data and material
All sequence reads are available from the NCBI SRA (PRJNA630450), and all other data files and results from analyses are available from the Dryad Digital Repository (https://doi.org/10.5061/dryad.brv15dv6s).
References
Alexandrino J, Baird SJ, Lawson L et al (2005) Strong selection against hybrids at a hybrid zone in the Ensatina ring species complex and its evolutionary implications. Evolution 59:1334–1347
Bates D, Maechler M, Bolker B et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bayona-Vásquez NJ, Glenn TC, Kieran TJ et al (2019) Adapterama III: Quadruple-indexed, double/triple-enzyme RADseq libraries (2RAD/3RAD). PeerJ 7:e7724
Beugin MP, Gayet T, Pontier D et al (2018) A fast likelihood solution to the genetic clustering problem. Methods Ecol Evol 9:1006–1016
Camp CD, Marshall JL, Landau KR et al (2000) Sympatric occurrence of two species of the two-lined salamander (Eurycea bislineata) complex. Copeia 2000:572–578
Camp CD, Wooten JA, Corbet CM et al (2013) Ecological interactions between two broadly sympatric, cryptic species of dusky salamander (genus Desmognathus). Copeia 2013:499–506
Catchen J, Hohenlohe PA, Bassham S et al (2013) Stacks: an analysis tool set for population genomics. Mol Ecol 22:3124–3140
Chatfield MWH, Kozak KH, Fitzpatrick BM et al (2010) Patterns of differential introgression in a salamander hybrid zone: inferences from genetic data and ecological niche modelling. Mol Ecol 19:4265–4282
Clark L (2017) lvclark/R_genetics_conv: R_genetics_conv 1.1. Zenodo. Available from https://doi.org/10.5281/zenodo.846816 (accessed April 2020)
de Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56:879–886
Devitt TJ, Baird SJ, Moritz C (2011) Asymmetric reproductive isolation between terminal forms of the salamander ring species Ensatina eschscholtzii revealed by fine-scale genetic analysis of a hybrid zone. BMC Evol Biol 11:245
Eaton DAR, Overcast I (2020) ipyrad: Interactive assembly and analysis of RADseq datasets. Bioinformatics 36:2592–2594
Fitzpatrick B, Placyk J Jr, Niemiller ML et al (2008) Distinctiveness in the face of gene flow: hybridization between specialist and generalist gartersnakes. Mol Ecol 17:4107–4117
Goodman SN (1999) Toward evidence-based medical statistics. 2: the bayes factor. Ann Intern Med 130:1005–1013
Goudet J (2005) Hierfstat, a package for R to compute and test hierarchical F- statistics. Mol Ecol Notes 5:184–186
Guttman SI, Karlin AA (1986) Hybridization of cryptic species of two-lined salamanders (Eurycea bislineata complex). Copeia 1986:96–108
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297
Harrison RG (1990) Hybrid zones: windows on evolutionary process. Oxford Surv Evol Biol 7:69–128
Hewitt GM (1988) Hybrid zones—natural laboratories for evolutionary studies. Trends Ecol Evol 3:158–549167
Highton R, Peabody RB (2000) Geographic protein variation and speciation in salamanders of the Plethodon jordani and Plethodon glutinosus complexes in the southern Appalachian Mountains with the description of four new species. In: Bruce RC, Jaeger RG, Houck LD (eds) The Biology of Plethodontid Salamanders. Springer, New York, pp 31–93
Hoffberg SL, Kieran TJ, Catchen JM et al (2016) RADcap: sequence capture of dual-digest RADseq libraries with identifiable duplicates and reduced missing data. Mol Ecol Res 16:1264–1278
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Howell T, Switzer V (1953) Intergrades of the Two-Lined Salamander, Eurycea bislineata, in Georgia. Herpetologica 9:152
Jacobs JF (1987) A preliminary investigation of geographic variation and systematics of the two-lined salamander, Eurycea bislineata (Green). Herpetologica 43:423–446
Jaeger RG (1971) Competitive exclusion as a factor influencing the distributions of two species of terrestrial salamanders. Ecology 52:632–637
Jockusch EL, Wake DB (2002) Falling apart and merging: diversification of slender salamanders (Plethodontidae: Batrachoseps) in the American West. Biol J Lin Soc 76:361–391
Jombart T, Ahmed I (2011) adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27:3070–3071
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc 90:773–795
Kozak KH (2003) Sexual isolation and courtship behavior in salamanders of the Eurycea bislineata species complex, with comments on the evolution of the mental gland and pheromone delivery behavior in the Plethodontidae. Southeast Nat 2:281–292
Kozak KH, Montanucci RR (2001) Genetic variation across a contact zone between montane and lowland forms of the two-lined salamander (Eurycea bislineata) species complex: a test of species limits. Copeia 2001:25–34
Kozak KH, Blaine RA, Larson A (2006) Gene lineages and eastern North American palaeodrainage basins: phylogeography and speciation in salamanders of the Eurycea bislineata species complex. Mol Ecol 15:191–207
Kuchta SR, Parks DS, Mueller RL et al (2009) Closing the ring: historical biogeography of the salamander ring species Ensatina eschscholtzii. J Biogeogr 36:982–995
Linck E, Battey C (2019) Minor allele frequency thresholds strongly affect population structure inference with genomic datasets. Mol Ecol Res 19:639–647
MacArthur RH, Wilson EO (1967) The Theory of Island Biogeography. Princeton University Press, Princeton
Marshall JL (2000). Alternative mechanisms of character divergence: theoretical and empirical consideration. Dissertation, University of Louisiana at Lafayette
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Petranka JW (1998) Salamanders of the United States and Canada. Smithsonian Institution Press, Washington DC
Pierson TW (2019). Organization of genetic and reproductive behavioral diversity in the two-lined salamander (Eurycea bislineata) species complex. Dissertation, University of Tennessee Knoxville
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Sattler PW, Brophy TR (2019) Distribution, Hybridization, and Taxonomic Status of Two-lined Salamanders (Eurycea bislineata complex) in Virginia and West Virginia. Banisteria 52:28–36
Sever DM, Dundee HA, Sullivan CD (1976) A new Eurycea (Amphibia: Plethodontidae) from southwestern North Carolina. Herpetologica 32:26–29
Wake DB (2006) Problems with species: patterns and processes of species formation in salamanders. Ann Mo Bot Gard 93:8–23
Wake DB (2009) What salamanders have taught us about evolution. Annu Rev Ecol Evol Syst 40:333–352
Walls SC (2009) The role of climate in the dynamics of a hybrid zone in Appalachian salamanders. Glob Change Biol 15:1903–1910
Weisrock DW, Hime PM, Nunziata SO et al (2018) Surmounting the large-genome “problem” for genomic data generation in salamanders. In: Hohenlohe P, Rajora OP (eds) Population Genomics. Springer, New York, pp 1–28
Acknowledgements
We thank Ken Kozak for aiding with the initial sample collection for the design of RADcap loci and for general guidance throughout this research. We thank Natalia Bayona-Vásquez, Joel Corush, Allison Devault, Travis Glenn, and Troy Kieran for aid in experimental design and laboratory assistance. We thank Natalia Bayona-Vásquez, Cyndi Carter, Troy Kieran, and Alexander Miele for aid in the field.
Funding
Funding was provided by the North Carolina Herpetological Society and the University of Tennessee Knoxville’s Department of Ecology and Evolutionary Biology Research Funds, and TWP was supported by a National Science Foundation Graduate Research Fellowship (DGE-1452154).
Author information
Authors and Affiliations
Contributions
All authors conceived of and designed the study and analyses. TWP and CDC collected data in the field. TWP conducted all laboratory work, analyzed data, and wrote the manuscript. All authors edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest or competing interests.
Ethical approval
This research was approved under an animal use protocol from the University of Tennessee Knoxville IACUC (#2482-0916), and collections were permitted by the Chattahoochee National Forest and in compliance with all local, state, and federal wildlife laws and regulations. The authors declare no conflicts of interest.
Consent for publication
All authors approved the final version of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pierson, T.W., Fitzpatrick, B.M. & Camp, C.D. Genetic data reveal fine-scale ecological segregation between larval plethodontid salamanders in replicate contact zones. Evol Ecol 35, 309–322 (2021). https://doi.org/10.1007/s10682-020-10099-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-020-10099-1