Abstract
We used both satellite tracking and carbon, nitrogen and sulphur stable isotopic analysis (SIA) to infer wintering ecology and habitat use of the Corsican osprey Pandion haliaetus population. A control sample of feathers from 75 individuals was collected within the osprey’s northern hemisphere breeding range, to assess the SIA variability across habitat types. An experimental set of SIA on feathers of 18 Corsican adults was examined to infer wintering ground locations and habitat types used during the non-breeding period. We calibrated the SIA using GPS/GSM tracks of 12 Mediterranean adults’ movements as wintering site references. We found 50% of individuals were resident and the other half migrated. Ospreys spent the winter at temperate latitudes and showed a high plasticity in habitat selection spread over the Mediterranean basin (marine bays, coastal lagoons/marshland, inland freshwater sites). Complementary to GPS tracking, SIA is, at a broad geographical scale, a reliable method to determine whether ospreys overwinter in a habitat different from that of their breeding area. This study proved that the integration of SIA and GPS/GSM tracking techniques was effective at overcoming the intrinsic limits of each method and achieving greater information for basic ecological studies of migratory birds in aquatic environments.
Similar content being viewed by others
Introduction
Animal population dynamics are dependent on a series of factors, which occur during different periods of the annual cycle (Newton 2003). To study migratory birds, it is therefore mandatory to understand the ecology of the species at each biological phase (e.g., breeding, migration and wintering). For decades, migratory movements of birds have been studied by means of ring-recovery data (Baillie et al. 2009). Advances in technology and chemistry provided tools for gathering essential information on individual movements and connectivity between breeding, wintering and stopover sites (Webster et al. 2002; Trierweiler et al. 2014; Choi et al. 2020). Satellite (GPS) tracking reveals wintering grounds and the migratory routes (tracks) followed between breeding and non-breeding areas with high accuracy and precision (Phipps et al. 2019; Sarà et al. 2019). Carbon, Nitrogen, Sulphur, Deuterium and Oxygen Stable Isotope Analyses (SIA), measured in feathers or other tissues, allowed further determination of seasonal habitat use and diet (Hobson 1999; Atkinson et al. 2005; Blight et al. 2014; Hobson and Wassenaar 2018). The rationale of SIA relies on tissues like feathers, which integrate chemical elements that are ingested through the diet during the growth period (post-moult) and do not change their composition over time (Hobson 1999; Ramos et al. 2009). This technique has the advantage of being minimally intrusive and relatively cheap (Hobson 1999). Combining these two techniques allowed collecting comprehensive information at the population level, improving the understanding of marine and terrestrial populations’ year-round ecology (Bentaleb et al. 2011) a crucial cue for planning adequate management measures at the appropriate scale (Inger and Bearhop 2008; Vander Zanden et al. 2018).
Isotopes of hydrogen and oxygen are typically used to explore movement ecology of terrestrial animals because of the well-known rainwater latitudinal patterns (Bowen 2010; Hobson and Wassenaar 2018). However, these isotopes function poorly for exploring the movement ecology of marine organisms or organisms that feed principally in aquatic/marine environments, especially over reduced spatial scales, because of the relatively low range of both hydrogen and oxygen stable isotope values in marine environments (e.g., Trueman and St. John Glew 2019). This may specifically affect the study of species living in strict association with marine or coastal environments, like seabirds (Bond and Jones 2009), where high spatial resolution reference maps of the isotopic landscape are missing (i.e., isoscapes; West et al. 2010). Among the other isotopic ratios, carbon and nitrogen (δ13C, δ15N) have been more frequently used as they are acknowledged as good indicators of foraging ecology patterns in terrestrial and aquatic environments (Hobson 1999; Bentaleb et al. 2011; Ceia et al. 2015). δ13C of primary producers vary predictably among ocean basins, with high-latitude pelagic ecosystems having much lower δ13C than similar ecosystems at lower latitudes (e.g.,Bentaleb et al. 1998; Cherel and Hobson 2007; Authier et al. 2012). δ15N of primary producers reflects the nitrogen cycle and the nitrate demand, varying with respect to marine productivity (e.g., Goericke and Fry 1994; Altabet et al. 1999). Knowing the baseline nitrogen isotopic value allow theoretically the prediction of the δ15N signatures of components of a specific trophic chain applying an N isotopic fractionation of 3.4 per mil (DeNiro and Epstein 1971). However, different processes such as ontogenetic developments for mammals (e.g., milk consumption; Authier et al. 2011) need to be assessed in some cases. More recent studies in ecology show an increasing interest on Sulphur isotopic ratios (δ34S) as they represent an efficient tool to discriminate between freshwater and coastal/marine habitats (Hobson 1999; Knoff 2000; Caccamise et al. 2000; Lott et al. 2003). Even though this approach does not provide exact determination of wintering ground location, it allows evaluating patterns of habitat use. Combining GPS acquired movement data may solve this issue and further enhance the winter ecology understanding of target species.
For this reason, to explore the wintering ecology of the osprey Pandion haliaetus, our target species, we relied on both GPS tracking and stable multi-isotopes of carbon, nitrogen and sulphur. The osprey is the only piscivorous diurnal raptor, of necessity living in strict association with aquatic environments (Cramp and Simmons 1980). In the Mediterranean basin, breeding sites are mostly located on the rocky cliffs of the islands and in coastal wetlands (Monti et al. 2020). GPS tracking studies revealed that Mediterranean ospreys are partial migrants and differ from their conspecifics belonging to northern central European populations, with respect to both temporal (total migration speed and use of stopovers) and spatial (distance, routes and main direction) components of migration strategy (Monti et al. 2018a). The great majority of Mediterranean ospreys wintered within the Mediterranean basin, spending the winter in southern Spain, Mediterranean islands and northern Africa. In some cases, some individuals behave as residents, staying at breeding grounds all year round (Monti et al. 2018b). Only a few individuals from the Mediterranean region winter in sub-Saharan Africa, as their conspecifics from northern Europe commonly do (Monti et al. 2018a).
In this study, we attempted to understand the range and feeding habitat of Mediterranean ospreys during winter using stable isotopes analyses (SIA) of feathers for a larger sample of individuals than is available from satellite tracked birds, to infer winter range and habitat use at the population level. A secondary aim of the study was to test whether SIA was a reliable tool for inferring wintering ground locations and habitat types used during the non-breeding period without deploying additional telemetry tags (since the costs of GPS devices is high, potentially limiting the sample size of tracked individuals). Because ospreys generally moult their body and flight feathers in winter (Prevost 1983; Electronic Supplementary Material S1), we expect that the isotopic chemical composition of the feathers reflects that of the habitat used in winter. Therefore, differences in stable isotopic ratios present in feathers would help determining if individuals wintered at different latitudes and foraged in different habitats (e.g., Bearhop et al. 1999; Inger and Bearhop 2008). To assess the isotopic method we used the GPS tracking data of ospreys’ movements as a reference for migratory routes and wintering sites’ location and habitat, allowing calibration of the isotopic composition with wintering locations.
Therefore, we expect these isotopic ratio values in osprey feathers to be potential proxies (a) to investigate latitudinal variation (Kelly et al. 2002; Farmer et al. 2004) and locate ospreys’ wintering grounds at a broad geographical scale; and (b) to link aquatic habitat types to different foraging areas in winter (Hobson 1999; Romanek et al. 2000).
Materials and methods
Study species
The osprey is a medium-size raptor species tightly linked to aquatic habitats, with a cosmopolitan distribution (Poole 2019). In the Western Palearctic, its breeding range stretches from Arctic countries like Finland and Norway to temperate Europe, where it breeds close to freshwater rivers and lakes (Cramp and Simmons 1980). At lower latitudes, as in the Mediterranean islands and North African coasts, as well as in Canary and Cape Verde archipelago, the osprey breeds in marine environments and coastal wetlands (Siverio et al. 2018; Monti et al. 2018a). Wintering birds also live close to marine environments along the Atlantic coast of Africa. Depending on latitude, reproduction generally starts in March–April; eggs hatch in April–May and chicks fledge in June-July. Birds depart in migration in August across a large front, eventually crossing the Mediterranean Sea (Duriez et al. 2018). They remain at wintering sites from October to February, where they moult their flight feathers (Prevost 1983). Just before spring migration (February), ospreys generally stop moulting after having undergone a complete or nearly complete moult of the primaries (Prevost 1983—Electronic Supplementary Material S1).
GPS tracking
Wintering ecology was assessed for 12 adult ospreys from the Mediterranean population, including 5 birds from Balearic Islands and 7 from Corsica (Electronic Supplementary Material S2). Five adults were trapped during both summer and winter seasons in the Albufera wetlands (Mallorca Island, Balearics), using a perch-trap (e.g., Prevost 1984). These birds were fitted with 30-gr Solar Argos/GPS PTT-100 s (Microwave Telemetry Inc., USA). An additional seven adults (five females and two males) were caught along the western coast of Corsica, France, before the onset of the breeding season in March–April 2013, using a noose carpet laid on the nest. These individuals were equipped with a 24-g solar powered GPS/GSM device (model Duck-4, Ecotone, Poland). These seven Corsican individuals were also sampled for stable isotopes analyses (see below), and used as a validation sample.
All tags were programmed to collect data at hourly intervals during the whole winter season. For migratory birds, the wintering season was defined as the period between the arrival on the wintering grounds after the post-breeding migration and the onset of the next pre-breeding migration (following Strandberg et al. 2008; Monti et al. 2018b). For resident birds, winter was considered as spanning from October to February, according to the biology of osprey at these latitudes (Poole 1989; Monti et al. 2018b). GPS locations were imported into QGIS (v. 3.6.1) and projected to the Universal Transverse Mercator (UTM) coordinate system for all spatial analyses.
Seven birds were tracked for more than 2 consecutive years, providing a total of 20 wintering events (Electronic Supplementary Material S2). However, to avoid any pseudo-replication bias during analyses, for individuals from Balearics, we systematically selected only the most recent wintering event. This approach was the most conservative to maximize the chance to record an adult non-breeding home range instead of an immature (exact birds’ age at ringing was unknown). For Corsican adult birds, we considered the first wintering event that was the temporally closest when feathers have been sampled for isotopes analyses. Our dataset was hence composed of 12 individual wintering events. All GPS tracking data can be consulted in the Movebank data-repository (www.movebank.org; project study name: “Osprey in the Mediterranean”).
To describe the non-breeding areas, we estimated the individuals’ home ranges (95% kernel) and core areas (50% kernel) based on all winter GPS locations through fixed kernel density contours (sensu Worton 1989), using the Home Range App (https://shiny.cefe.cnrs.fr/HRApp/) through the R “adehabitatHR” package (Calenge 2011). Since ospreys are strictly associated with the presence of water bodies to catch fish (Cramp and Simmons 1980), we qualified habitat type composition during winter only according to aquatic environments: we calculated the percentage of marine water, brackish water or freshwater within the home range for each wintering event separately (see Monti et al. 2018b). This was achieved using satellite-images from Google Earth and available data in the literature as a source, together with local surveys, when feasible.
Stable isotope ecobiogeochemistry
Control sample
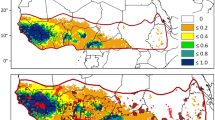
To have a baseline resolution of the isotopic variation over a large latitudinal gradient and between different habitat types within the osprey’s breeding range, we collected feathers from a control sample. Overall, we collected feathers belonging to 75 different individuals (65 nestlings and 10 adults), from 11 sites throughout the Western Palearctic: from Arctic Finland to Morocco and West Africa (Cape Verde islands). The nests sampled were located near three main aquatic habitats: (a) freshwater; (b) brackish water and (c) marine water (Table 1; Fig. 1a).
Maps with location of sites used for (a) feathers sampling (feathers of the control sample—black dots and numbers that refer to countries are reported in Table 1) and (b) wintering grounds of GPS tagged ospreys (black stars; see Electronic Supplementary Material S2)
Chicks’ feathers were collected during ringing actions at the nest in summer, to measure the isotopic values of the environment around breeding sites during breeding period. More specifically, we sampled body feathers of 30 chicks from Continental Europe (Finland, Estonia, Latvia and France; Table 1, Fig. 1a), where ospreys nest close to freshwater sites and chicks are mostly fed with stenohaline fish species (Poole 1989). We also sampled the body feathers of 29 chicks from the Mediterranean basin (Table 1): 26 from 3 osprey populations breeding on rocky pinnacles at sea (Corsica, Morocco and Balearics Islands), feeding on euryhaline species (Francour and Thibault 1996; Thibault et al. 2001) and 3 chick samples from coastal wetlands (e.g., in Italy, where chicks are fed with marine, brackish or freshwater fishes; Monti et al. 2020). We also included an additional six samples from chicks on Atlantic islands (Canary and Cape Verde islands) to have other marine isotopic signatures from lower latitudes in the Atlantic, to be compared with those of the Mediterranean area. We also added samples from adult ospreys wintering in West Africa (Senegal), to have a signature of wintering grounds used by northern European ospreys (Alerstam et al. 2006; Bai and Schmidt 2011). In this case, we collected a piece (about 2 cm length) of vexillum at the tip of primaries (actively moulted) from three specimens of adult osprey belonging to the museum collection of the IRD (Institut de la Recherche et du Développement of Dakar, Senegal; Table 1; Fig. 1a).
Experimental sample
For inferring whether adults breeding in Corsica use wintering grounds different from breeding areas, we sampled 18 adult ospreys trapped in March 2012 and 2013 at their nests in Corsica, before the onset of the breeding season (7 of these were also fitted with GPS loggers; see above). From these birds, we selected only recently moulted feathers (darker in colour and with more regular borders; Electronic Supplementary Material S1). For the analyses, we used only the first 2 cm of the tip of two primaries and two secondaries that had grown during the previous winter and, thus, contained the isotopic signature of wintering grounds (Electronic Supplementary Material S1).
Stable isotope analyses
Feathers belonging to 93 different individuals (75 control sample + 18 experimental sample) were analysed for stable isotopic ratios of Carbon (δ13C) and Nitrogen (δ15N) and 45 for Sulphur isotopic ratios (δ34S) (Table 1; Electronic Supplementary Material S1, S3 and S6). Sample size differs between C, N and S isotopic ratios because of limited quantity of feathers for certain individuals. Data for Nitrogen (N = 93) were not included in mixing models, as the number of isotopes should not be greater than n-1, n being the number of sources (see below; following Parnell et al. 2010). Moreover, while the osprey is a highly specialized piscivore, it is an opportunistic forager that can feed on a large variety of fish from a large range of trophic levels (Poole 1989), thus we expected large overlaps of Nitrogen values, even between different aquatic habitats (Electronic Supplementary Material S3 and S5). For these reasons, nitrogen isotopic results are not presented here but provided in Electronic Supplementary Material S3.
Feathers were thoroughly washed in distilled water using an ultra-sonic bath and then dried in an oven at 50 °C over night (Guillemain et al. 2014). Fragments of appropriate weight of feathers (for C = 0.3 mg, for S = 1 mg) were cut and placed in 8 × 5 mm EuroVector tin capsules. Carbon analysis of the Western Palearctic samples were conducted at the isotope platform of ISEM by means of a mass spectrometer Micromass Optima-AC117-coupled to an elemental analyser EuroVector 3000. Carbon analysis of the Western African samples were conducted at the isotopic platform LIENSs of University of La Rochelle using the isotope ratio mass spectrometer in continuous flow (CF-IRMS) Delta V Advantage, coupled with a Flash EA 1112 elemental analyser.
Stable isotope ratios were reported as deviations from a standard in per mil (‰) using the δ notation:
where δ is the isotope ratio of the sample relative to a standard, (i.e., 13C, 34S), Rsample and Rstandard are the fractions of heavy to light isotopes in the sample and standard, respectively (i.e., 13C/12C, 34S/33S).
The precision for C isotopic ratios is higher than 0.1‰ for both ISEM and LIENSs mass spectrometer devices. The C isotope of the alanine standards of the ISEM laboratory was measured on both ISEM and LIENSs machines showing no difference (−23.7‰). All the sulphur analyses were run at LIENSs laboratory. The precision was higher than 0.3‰.
As a preliminary methodological test, we analysed 30 feathers from 15 individuals (15 primaries and 15 secondaries) to assess whether isotopic signatures of C and N vary in relation to the type of feather (Zelanko et al. 2011). Because we found no significant differences in isotopic signatures of C and N between primary and secondary feather (Wilcoxon signed-rank test: δ13C: p = 0.24; δ15N: p = 0.40; N = 15), we used both feathers types for our analyses.
Statistical analysis
We first tested the hypothesis that feathers of control sample displayed significantly different isotopic signatures between the three habitats (freshwater, brackish water and marine water). Kruskall–Wallis tests were performed to analyse separately the differences in δ13C and δ34S ratios across habitats. A Dunn post hoc test was conducted when a significant difference was observed. To better discriminate habitat signatures, we performed permutational-MANOVA (PERMANOVA, adonis function in R) with 1000 permutations and using a Euclidean distance similarity index.
A common application of mixing models (Parnell et al. 2010) is to use the stable isotopic composition of individuals (usually called “consumers” in SIA) and their different type of foods (usually called “sources” in SIA) to make inferences about the composition of the animal’s assimilated diet. Here, we applied a mixing model to use isotopic signatures of Corsican adult ospreys (consumers) and ospreys from different putative habitats (sources) to infer wintering habitat of the former. Bayesian mixing models to solve relationships between organisms from stable isotope ratios models could show a bias toward a null generalist hypothesis when source estimates are inaccurate (Brett 2014), or when consumers fall outside the polygon bounding the proposed sources (i.e., when consumer isotopic values are not included in the range explained by the sources; Smith et al. 2013). We were particularly interested in the variance between age classes of Corsican ospreys because if a portion of the population is migratory, the range of isotopic values would increase the variance of that population. We used Student–Fischer test of equality of variance to compare values of Corsican adult ospreys and Corsican chicks and then, because the variance was not equal, we compared means with a Kruskall–Wallis test.
For application of the mixing model, samples from putative habitat were considered “sources”, and Corsican adult ospreys were considered “consumers”. As aforementioned, mixing models were firstly developed for inferring consumer diet from different sources considering uncertainty and trophic enrichment. There are some conceptual issues in the application of these tools to discriminate between habitats (Phillips et al. 2014). In particular, while a mixing model infers the diet of a consumer that can feed on multiple sources, this assumption could be wrong for habitat of Corsican ospreys visiting only one type of habitat. Following Taylor et al. (2016) and to identify consumers (adult Corsican ospreys) with isotopic values that were unlikely to be explained by the mixing model, we ran a stable isotope mixing polygon simulation (Smith et al. 2013) with 1500 iterations on the dataset. Corsican adult ospreys falling outside the resource polygon and having therefore a weak probability to be accurately assigned to a habitat were excluded from subsequent analyses (Electronic Supplementary Material S4). We ran a Bayesian mixing model (package simmr in R) with trophic enrichment factor set to zero. We assessed algorithm convergence using a Gelmann Diag diagnostic and checked the fit of the model with a posterior predictive check. Finally, to assess the reliability of the stable isotope analysis, we compared the habitat assigned by the mixing model with the habitats as observed for the seven individuals from Corsica that were also tracked by GPS and for which both winter location and habitat were exactly known.
Results
Differences in isotopic ratios between habitats
Carbon and Sulphur isotopic ratios significantly differed between habitats (δ13C: χ2 = 56.79, p < 0.001, df = 2; δ34S: χ2 = 19.71, p < 0.001, df = 2; Figs. 2, 3 and Electronic Supplementary Material S5). Dunn post hoc test results revealed that δ13C and δ34S values increased with salinity, being higher in marine habitat than in brackish (δ13C: Z = − 2.28, p = 0.022; δ34S: Z = − 3.13, p < 0.01), and freshwater habitats (δ13C: Z = − 7.52, p < 0.001; δ34S: Z = − 3.91, p < 0.001). Although isotope values were not significantly different between brackish and freshwater habitat (δ13C: Z = 1.84, p = 0.065; δ34S: Z = 0.65, p = 0.51; Fig. 2). However, PERMANOVA analysis revealed that the combination of the two isotopes differed significantly among habitats (F = 187.92, p < 0.001, df = 2). Moreover, pairwise PERMANOVA showed difference among all possible pairs of the three habitats (marine water vs freshwater: F = 402.81, p < 0.01, df = 1; marine water vs brackish water: F = 76.25, p < 0.01, df = 1; freshwater vs brackish water: F = 22.78, p < 0.01, df = 1).
Mean isotopic values for Corsican adults were significantly different from chicks for δ34S (t1 = 6.42, p < 0.001) but not for δ13C (t1 = 0.03, p = 0.857) (Fig. 4). Moreover, the variance between the two isotopes was higher in adults than in chicks (δ13C: t17 = 15.87, p < 0.001; δ34S: t17 = 11.04, p = 0.0316; Fig. 4).
GPS tracking: movements and habitat selection
Ten out of 12 adult birds tracked with GPS spent the winter at temperate latitudes. Their wintering grounds ranged between 28°N and 42°N latitude within the Mediterranean basin (e.g., Spain, Morocco, Algeria, Sardinia-Corsica complex, Balearics and Italy). The only two individuals that crossed the Sahara desert reached the Atlantic coast of southern Morocco and the Senegal River delta in Mauritania in 2009 (Electronic Supplementary Material S2). 50% of tagged individuals were residents (six individuals) and 50% (six individuals) migrated. Migratory ospreys spent about 6 months (mean: 172.7 ± 25.2 days, N = 6; Electronic Supplementary Material S2) at wintering grounds.
Adult ospreys generally used a unique site throughout the whole winter. Daily movements were restricted (mean = 12.11 ± 6.8 km per day) and exploratory movements were only occasional (e.g., an individual covered 140 km, coming back to its main wintering site on the same day). Home range sizes were very small during wintering period (home range = 58.25 ± 42.9 km2; core area = 7.25 ± 3.8 km2; Electronic Supplementary Material S2) and did not differ between resident and migratory birds (Mann–Whitney U Test: home range: U = 12, p = 0.37, N = 12; core areas: U = 14, p = 0.57, N = 12).
The birds tracked during consecutive wintering seasons showed a high inter-annual site-fidelity, as most of them used the same areas that they had frequented in the previous years (mean overlap home ranges: 41.8 ± 20.2%; N = 7; Electronic Supplementary Material S2).
In winter, adult ospreys used coastal marine water habitats (bays and coastal waters), brackish water habitats (estuaries) as well as freshwater sites in both coastal and inland areas (marshes, dams and artificial ponds). Inter-individual plasticity in habitat choice (calculated on 95% home ranges) was high, with 8.3% of the birds using only freshwater sites and 33.3% using exclusively brackish habitats such as marshland or coastal lagoons. The remaining 58.33% frequented different habitats during the same season (Table 1 and Electronic Supplementary Material S5). No bird has had a home range fully associated with the marine environment, but those who used different habitats have frequented marine and coastal areas.
Wintering habitats of experimental Corsican ospreys
To infer wintering location, the seven adults tracked by GPS, for which the exact wintering ground and habitat was known, were used as a validation sample. Stable isotope mixing polygon simulation showed that three consumers had isotopic values that were unlikely to be explained by the model and therefore a model solution was possible for 15 out of 18 (83%) of the Corsican adult ospreys. Thus, the remaining three (all not GPS-tagged birds) were excluded from Bayesian analysis (Electronic Supplementary Material S4).
For the remaining 15 Corsican adult ospreys, the mixing model identified marine water as the most frequented habitat, followed by brackish water and freshwater (Fig. 5). Each adult Corsican osprey revealed that the isotopic signature was characteristic of marine water, brackish water, or intermediate values between the two, but never close to freshwater isotopic values (Fig. 6). Five individuals exhibited typically marine isotopic signatures (birds 11–15 in Fig. 6; 95% Bayesian credibility intervals for estimated marine water contribution were higher than 65% and did not overlap other source contribution intervals), while one individual displayed isotopic signatures characteristic of a bird feeding almost only in brackish water (bird 3 in Fig. 6). Three other individuals fed mostly in marine habitat (birds 8–10 in Fig. 6) and three mostly in brackish water habitat (birds 2, 5 and 6 in Fig. 6). The remaining three Corsican adult ospreys (birds 1, 4 and 7) fed in variable combinations of the three habitats and dietary proportions were quite uncertain.
Predicted proportion for the contribution of the three supplied sources (freshwater in green, brackish water in yellow and marine water in blue) for each Corsican adult osprey isotopic signature. Reported values are the lower and upper 50% and 95% Bayesian credibility intervals predicted by the mixing models. For the seven individuals from Corsica that were also tracked by GPS (for whom precise wintering location and habitat composition is known) coloured bars represent the percentage of each habitat used from GPS tracks. ID of the GPS code is reported between brackets (see Electronic Supplementary Material S2)
Discussion
We performed a multi-isotopic analysis to determine the isotopic ratios and improve resolution in assessing osprey wintering ecology. Results from a control sample showed that the stable isotope ratios of osprey feathers varied greatly between different breeding habitats and latitudes in the Western Palearctic and West Africa. Significant differences in isotopic ratio efficiently allowed distinguishing chicks fed and reared in freshwater, brackish or marine environments over the latitudinal gradient. Results were concordant with the ecology of osprey breeding populations (populations nesting in northern Europe being more reliant on freshwater habitats while Mediterranean populations relying mostly on marine or brackish water habitats; Monti et al. 2020).
These trends allow the comparison of isotopic ratios in Corsican adult ospreys feathers with those of Corsican chicks fed in a marine habitat in the Mediterranean. The direct comparison of aquatic habitat composition available in the home range and the habitat predicted from SIA discriminant analysis for seven adult ospreys with known wintering sites revealed an overall good match between the two techniques (Fig. 6). The differences of habitats predicted by SIA and home range for two–three birds might be the result of a bird feeding in one habitat (e.g., seashore) but roosting in trees near freshwater. This would put freshwater habitat in the bird’s winter home range, even though it was not foraging in fresh water. Hourly GPS fixes are likely to miss foraging trips conducted between 2 consecutive hourly fixes. For example, a bird could be perched in a freshwater habitat at 1 hourly fix, fly out at close coastal wetlands or at sea, catch a fish, and be back to its perch before the next hourly fix (e.g., Electronic Supplementary Material S2). In addition, one should remember that the SIA was applied to feathers collected at the time of tagging the bird (i.e., moulted in the winter before the capture), while the home range analysis was performed in the winter after the capture. Thus, this calibration analysis was strongly relying on the assumption that adult ospreys are very faithful to their wintering area in subsequent years, as showed in Monti et al. (2018b) for the Mediterranean population, and as assessed in other populations across species’ distribution range (Washburn et al. 2014; Poole 2019). However, we cannot exclude the possibility that some birds may have used different wintering grounds before and after the capture, resulting in a mismatch between the two methods. Overall SIA discriminant analysis seems to be a powerful method to determine habitat used in winter for ospreys (and possibly other migratory waterbirds) that were not tracked by GPS.
The combined use of SIA and GPS tracking confirmed the partial migratory behaviour of the Mediterranean population. However, a potential bias in our study could be related to the fact that we used some birds trapped in winter in the Balearics (see methods); that might have increased the likelihood of capturing sedentary individuals, since migratory ones may have already left at that time of capture. The adult Corsican ospreys, captured in spring before reproduction, are probably more representative of the proportion of sedentary and migratory birds than the Balearic sample.
Nonetheless, we recorded a high plasticity in wintering strategies of Mediterranean ospreys. Even if it were impossible to determine exactly the latitude of each wintering area, SIA revealed that the majority of Corsican ospreys overwinter in a habitat different from their breeding habitat. Only 33% (5 out of 15 individuals) of Corsican adult feathers had isotopic values similar to those of Corsican chicks (marine habitat), suggesting a similar proportion of residents in this population. This resident behaviour in the Mediterranean osprey population was confirmed by GPS tracking (50% of tagged birds did not migrate). The remaining individuals showing different isotopic values could suggests three potential outcomes: (1) adults get all of their isotope sources from local habitats on the wintering grounds—in that case these adults had most likely spent the winter in different habitats (such as brackish and freshwater sites) located away from Corsica, because such sites are rare in Corsica (but more common in the neighbouring Sardinia for example) and are rarely, if ever, used by ospreys in winter (Thibault et al. 1996); (2) the adults actually use different isotopic sources, that stored from fat and other tissues (capital) as well as that from their diet (income), to grow their feathers, thus differing from that of the chicks; (3) there might be some differences in how growing chicks metabolically discriminate between heavy and light isotopes relative to the adults.
Furthermore, low variances in δ13C and in δ34S within each breeding area suggested that the source of organic matter in the food web was similar for all sampled chicks (e.g., marine prey during breeding season). This is in accordance with the strictly piscivorous habits of the species and the limited movements recorded during the breeding period (Monti et al. 2018c). Then, despite the small sample size, the lack of a significant difference in isotopic ratios between adults (mean δ13C: − 15.1 ± 1.1; mean δ15N: 12.4 ± 0.5; N = 7) and chicks (mean δ13C: −16.2 ± 0.6; mean δ15N: 11.5 ± 0.6; N = 2) from the Cape Verde islands also suggests that adults in this population are mostly resident, as reported in the literature (Poole 1989). However, this cannot be a conclusive evidence that chicks and resident adults have similar isotopic signatures owing to feeding on similar resources, especially because a test of the difference cannot be applied in this case.
Mediterranean ospreys tracked in winter mostly used temperate areas, but different habitat types (from marine bays to marshlands and, to a lesser extent, freshwater sites). While on one hand a high inter-individual variability was detected in the habitat used in winter within the Mediterranean population, on the other hand, each adult individual tended to use only one site (rarely two). This general lack of mobility in winter supported and indeed strengthened our assumptions made with SIA. Once arrived at the wintering ground, adult birds rarely moved around, but rather exploited a small area associated with a specific water body (Monti et al. 2018b).
This study proved that the integration of multiple SIA and tracking techniques can be useful to overcome the intrinsic limits of each method and achieve greater information about the basic ecology of migratory birds. The main limitation of SIA was the correlation between latitude of the sampling site and habitat type for carbon that prevented us from discerning the exact latitude of wintering grounds. For example, δ13C and δ34S values for samples from Senegal were equivalent to those recorded for Italy, where ospreys fish mostly in brackish water habitats in both regions. The downside of GPS tracking is obviously its cost, limiting the sample size, but compensated by a high resolution to determine wintering sites and movements in winter. This study showed that using SIA together with GPS tracking provides strong insights on the trophic level and shifts in diet composition in migratory birds wintering in aquatic habitats and where both stenohaline and euryhaline prey species can be present.
References
Alerstam T, Hake M, Kjellén N (2006) Temporal and spatial patterns of repeated migratory journeys by ospreys. Anim Behav 71:555–566. https://doi.org/10.1016/j.anbehav.2005.05.016
Altabet MA, Pilskaln C, Thunell R, Pride C, Sigman D, Chavez F, Francois R (1999) The nitrogen isotope biogeochemistry of sinking particles from the margin of the Eastern North Pacific. Deep-Sea Res 46:655–679. https://doi.org/10.1016/S0967-0637(98)00084-3
Atkinson PW, Baker AJ, Bevan RM, Clark NA, Cole KB, Gonzalez PM, Newton J, Niles LJ, Robinson RA (2005) Unravelling the migration and moult strategies of a long-distance migrant using stable isotopes: red knot Calidris canutus movements in the Americas. Ibis 147:738–749. https://doi.org/10.1111/j.1474-919x.2005.00455.x
Authier M, Martin C, Ponchon A, Steedlandt S, Bentaleb I, Guinet C (2011) Breaking the sticks: a hierarchical change-point model for estimating ontogenetic shifts with stable isotope data. Methods Ecol Evol 3(2):281–290. https://doi.org/10.1111/j.2041-210X.2011.00162.x
Authier M, Bentaleb I, Ponchon A, Martin C, Guinet C (2012) Foraging strategy and longevity in male Southern elephant seals. PLoS ONE 7(4):e32026
Bai ML, Schmidt D (2011) Differential migration by age and sex in central European Ospreys Pandion haliaetus. J Ornithol 153:75–84. https://doi.org/10.1007/s10336-011-0697-y
Baillie SR, Robinson RA, Clark JA, Redfern CP (2009) From individuals to flyways: the future of marking birds for conservation. Ringing Migr 24:155–161. https://doi.org/10.1080/03078698.2009.9674386
Bearhop S, Thompson DR, Waldron S, Russell IC, Alexander G, Furness RW (1999) Stable isotopes indicate the extent of freshwater feeding by cormorants Phalacrocorax carbo shot at inland fisheries in England. J Appl Ecol 36:75–84. https://doi.org/10.1046/j.1365-2664.1999.00378.x
Bentaleb I, Fontugne M, Descolas-Gros C, Girardin C, Mariotti A, Pierre C, Brunet C, Poisson A (1998) Carbon isotopic fractionation by plankton in the Southern Indian Ocean: relationship between δ13C of particulate organic carbon and dissolved carbon dioxide. J Mar Syst 17:39–58. https://doi.org/10.1016/S0924-7963(98)00028-1
Bentaleb I, Martin C, Mate B, Mayzaud P, Siret D, de Stephanis R, Guinet C (2011) Foraging shift of Mediterranean fin whales in a changing environment elucidated by satellite tracking and baleen plate stable isotopes. Mar Ecol Prog Ser 438:285–302. https://doi.org/10.3354/meps09269
Blight LK, Hobson KA, Kyser TK, Arcese P (2014) Changing gull diet in a changing world: A 150-year stable isotope (δ13C, δ15N) record from feathers collected in the Pacific Northwest of North America. Glob Change Biol 21:1497–1507. https://doi.org/10.1111/gcb.12796
Bond AL, Jones IL (2009) A practical introduction to stable isotope analysis for seabird biologists: approaches, cautions and caveats. Marine Ornithol 37:183–188
Bowen GJ (2010) Isoscapes: Spatial pattern in isotopic biogeochemistry. Annu Rev Earth Planet Sci 38:161–187. https://doi.org/10.1146/annurev-earth-040809-152429
Brett MT (2014) Resource polygon geometry predicts bayesian stable isotope mixing model bias. Mar Ecol Prog Ser 514:1–12. https://doi.org/10.3354/meps11017
Caccamise DF, Reed LM, Castelli PM, Wainright S, Nichols TC (2000) Distinguishing migratory and resident Canada geese using stable isotope analysis. J Wildl Manag 64:1084
Calenge C (2011) Home Range Estimation in R: The adehabitatHR. Package R package version 0.4.4. The Comprehensive R Archive Network. Project.org/web/packages/adehabitatHR/index.html
Ceia FR, Ramos JA, Phillips RA, Cherel Y, Jones DC, Vieira RP, Xavier JC (2015) Analysis of stable isotope ratios in blood of tracked wandering albatrosses fails to distinguish a δ13C gradient within their winter foraging areas in the southwest Atlantic Ocean. Rapid Commun Mass Spectrom 29:2328–2336. https://doi.org/10.1002/rcm.7401
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287. https://doi.org/10.3354/meps329281
Choi CY, Nam HY, Park JG, Bing GC (2020) Migration pattern of Yellow-throated buntings revealed by isotope-based geographic assignment. Internat J Geo Inform Sci 34(3):504–519. https://doi.org/10.1080/13658816.2019.1670832
Cramp S, Simmons KEL (1980) Handbook of the birds of Europe, the Middle East and North Africa The birds of the Western Palearctic. Oxford University Press, Oxford
Duriez O, Péron G, Grémillet D, Sforzi A, Monti F (2018) Migrating ospreys use thermal uplifts over the open sea. Biol Lett 14(12):10. https://doi.org/10.1098/rsbl.2018.0687
Farmer A, Abril M, Fernández M, Torres J, Kester C, Bern C (2004) Using stable isotopes to associate migratory shorebirds with their wintering locations in Argentina. Ornitol Neotr 15:377–384
Francour P, Thibault JC (1996) The diet of breeding Osprey Pandion haliaetus on Corsica: exploitation of a coastal marine environment. Bird Study 43:129–133. https://doi.org/10.1080/00063659609461004
Goericke R, Fry B (1994) Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochem Cycles 8:85–90. https://doi.org/10.1029/93GB03272
Guillemain M, Wilgenburg SL, Legagneux P, Hobson KA (2014) Assessing geographic origins of Teal (Anas crecca) through stable-hydrogen (δ2H) isotope analyses of feathers and ring-recoveries. J Ornithol 155:165–172. https://doi.org/10.1007/s10336-013-0998-4
Hobson KA (1999) Tracing diets and origins of migratory birds using stable isotope techniques. Society of Canadian Ornithologists Special Publication, Fredericton, New Brunswick
Hobson KA, Wassenaar LI (2018) Tracking animal migration with stable isotopes. Academic Press, Newyork
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461. https://doi.org/10.1111/j.1474-919X.2008.00839.x
Kelly JF, Atudorei V, Sharp ZD, Finch DM (2002) Insights into Wilson’s Warbler migration from analyses of hydrogen stable-isotope ratios. Oecologia 130:216–221. https://doi.org/10.1007/s004420100789
Knoff AJ (2000) Stable isotope analysis of intrapopulation, spatial, and temporal variation of Laughing Gull (Larus atricilla) diets in the virginia coast reserve and Jamaica Bay New York - Unpublished M.Sc. University of Virginia, Virginia
Lott CA, Meehan TD, Heath JA (2003) Estimating the latitudinal origins of migratory birds using hydrogen and sulfur stable isotopes in feathers: influence of marine prey base. Oecologia 134:505–510. https://doi.org/10.1007/s00442-002-1153-8
Monti F, Grémillet D, Sforzi A, Dominici JM, Triay Bagur R, Muñoz Navarro A, Fusani L, Klaassen RHG, Alerstam T, Duriez O (2018a) Migration distance affects stopover use but not travel speed: Contrasting patterns between long- and short-distance migrating ospreys. J Avian Biol 49:e01839. https://doi.org/10.1111/jav.01839
Monti F, Grémillet D, Sforzi A, Sammuri G, Dominici JM, Triay Bagur R, Muñoz Navarro A, Fusani L, Duriez O (2018b) Migration and wintering strategies in vulnerable mediterranean osprey populations. Ibis 160:554–567. https://doi.org/10.1111/ibi.12567
Monti F, Duriez O, Dominici JM, Sforzi A, Robert A, Fusani L, Grémillet D (2018c) The price of success: integrative long-term study reveals ecotourism impacts on a flagship species at a UNESCO site. Anim Conserv 21:448–458. https://doi.org/10.1111/acv.12407
Monti F, Bianchi N, Sforzi A, Leonzio C, Ancora S (2020) Drawing the baseline of trace element levels in the vulnerable Mediterranean osprey Pandion haliaetus: variations by breeding location, habitats, and egg components. Environ Sci Pollut Res 27(10):10236–10248. https://doi.org/10.1007/s11356-019-07591-9
Newton I (2003) Population limitation in birds. Academic Press, London
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672. https://doi.org/10.1371/journal.pone.0009672
Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835. https://doi.org/10.1139/cjz-2014-0127
Phipps WL, López-López P, Buechley ER, Oppel S, Álvarez E, Arkumarev V, Bekmansurov R, Berger-Tal O, Bermejo A, Bounas A, Alanís IC, de la Puente J, Dobrev V, Duriez O, Efrat R, Fréchet G, García J, Galán M, García-Ripollés C, Gil A, Iglesias-Lebrija JJ, Jambas J, Karyakin IV, Kobierzycki E, Kret E, Loercher F, Monteiro A, Morant Etxebarria J, Nikolov SC, Pereira J, Peške L, Ponchon C, Realinho E, Saravia V, Şekercioğlu ÇH, Skartsi T, Tavares J, Teodósio J, Urios V, Vallverdú N (2019) Spatial and temporal variability in migration of a soaring raptor across three continents. Front Ecol Evol 7:1–14. https://doi.org/10.3389/fevo.2019.00323
Poole AF (1989) Ospreys: a Natural and Unnatural History. Cambridge University Press, Cambridge
Poole AF (2019) Ospreys: the revival of a global raptor. Johns Hopkins University Press, Baltimore, MD
Prevost YA (1983) The moult of the Osprey. Ardea 71:199–209
Prevost YA (1984) A perch snare for catching ospreys. J Wildlife Manage 48:991–993. https://doi.org/10.2307/3801454
Ramos R, González-Solís J, Ruiz X (2009) Linking isotopic and migratory patterns in a pelagic seabird. Oecologia 160:97–105. https://doi.org/10.1007/s00442-008-1273-x
Romanek CS, Gaines KF, Alb J (2000) Foraging ecology of the endangered wood stork recorded in the stable isotope signature of feathers. Oecologia 125:584–594. https://doi.org/10.1007/s004420000471
Sarà M, Bondì S, Bermejo A, Bourgeois M, Bouzin M et al (2019) Broad-front migration leads to strong migratory connectivity in the lesser kestrel Falco naumanni. J Biogeogr 46:2663–2677. https://doi.org/10.1111/jbi.13713
Siverio M, Siverio F, Rodríguez B, Del Moral JC (2018) El águila pescadora en España y Portugal: población invernante 2016–2017, reproductora en 2018 y método de censo. SEO/BirdLife, Madrid
Smith JA, Mazumder D, Suthers IM, Taylor MD (2013) To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods Ecol Evol 4:612–618. https://doi.org/10.1111/2041-210X.12048
Strandberg R, Klaassen RH, Hake M, Olofsson P, Thorup K, Alerstam T (2008) Complex timing of Marsh Harrier Circus aeruginosus migration due to pre-and post-migratory movements. Ardea 96:159–171. https://doi.org/10.5253/078.096.0202
Taylor MD, Smith JA, Boys CA, Whitney H (2016) A rapid approach to evaluate putative nursery sites for penaeid prawns. J Sea Res 114:26–31. https://doi.org/10.1016/j.seares.2016.05.004
Thibault JC, Triay R, Beabrun P, Boukhalfa D, Dominici JM, Torre A (1996) Osprey (Pandion haliaetus) in the Mediterranean: characteristics of a resident population with a patchy distribution. In: Muntaner J, Mayol J (eds) Biologia y Conservacion de las Rapaces Mediterraneas Monografias, no 4. SEO, Madrid
Thibault JC, Bretagnolle V, Dominici JM (2001) Le Balbuzard pêcheur en Corse: du martyre au symbole de la protection de la nature. Alain Piazzola, Ajaccio
Trierweiler C, Klaassen RHG, Drent RH, Exo KM, Komdeur J, Bairlein F, Koks BJ (2014) Migratory connectivity and population-specific migration routes in a long-distance migratory bird. Biol Sci 281:20132897–20132897. https://doi.org/10.1098/rspb.2013.2897
Trueman CN, St John Glew KL (2019) Isotopic tracking of marine animal movement. In: Hobson K, Wassenaar LI (eds) Tracking animal migration using stable isotopes. Academic Press, London, UK
Vander Zanden HB, Nelson DM, Wunder MB, Conkling TJ, Katzner T (2018) Application of isoscapes to determine geographic origin of terrestrial wildlife for conservation and management. Biol Cons 228:268–280. https://doi.org/10.1016/j.biocon.2018.10.019
Washburn BE, Martell MS, Bierregaard RO, Henny CJ, Dorr BS, Olexa TJ (2014) Wintering ecology of adult North American Ospreys. J Raptor Res 48:325–333. https://doi.org/10.3356/JRR-OSPR-13-01.1
Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83. https://doi.org/10.1016/S0169-5347(01)02380-1
West JB, Bowen GJ, Dawson TE, Tu KP (2010) Isoscapes: understanding movement, pattern, and process on earth through isotope mapping. Springer, New York, p 487
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168. https://doi.org/10.2307/1938423
Zelanko PM, Rice NH, Velinsky DJ (2011) Using carbon and nitrogen stable isotopes to distinguish the locations of feather growth in osprey (Pandion haliaetus). Proc Acad Nat Sci Phila 161:61–72. https://doi.org/10.2307/41445994
Acknowledgments
We thank all the colleagues that kindly provided osprey samples for this study: Pertti Saurola, Harri Koskinen and Juhani Koivu (Finland), Urmas Sellis (Estonia), Aigars Kalvāns (Latvia), Rolf Wahl (France), Manuel Siverio (Canary Islands), Pedro López-Suárez (Cape Verde) and Houssine Nibani (Morocco). Many thanks are also addressed to Georges de Noni and Daouda Sylla from the IRD (Institut de la Recherche et du Développement of Dakar, Senegal) that allowed us to collect samples from their collection. This study was funded thanks to a grant from the Foundation Prince Albert II de Monaco and the Associazone italiana della Fondation Prince Albert II de Monaco ONLUS. It also benefits from the support of the Parc Naturel Régional de Corse, Maremma Regional Park and Conservatoire du Littoral (through the Mediterranean Small Islands Initiative PIM). F. Monti was supported by a grant of the Maremma Regional Park (Italy) and by a mobility grant of the Università Italo Francese/Université Franco Italienne (UIF/UFI). David Grémillet, Leonida Fusani and Maëlle Connan greatly helped with comments and suggestions on a first draft of this article.
Funding
Open Access funding provided by Università degli Studi di Siena.
Author information
Authors and Affiliations
Contributions
FM, OD and IB conceived and designed the experiments. FM, AR, JMD, AS, RFT, AMN and OD did the fieldwork. FM and OD analysed GPS tracking data. AR and IB prepared feathers for analyses and analysed stable isotopic data. GG helped with analytical data in the laboratory. FM, AR and OD wrote the manuscript. All authors contributed critically to the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Seth Newsome.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monti, F., Robert, A., Dominici, JM. et al. Using GPS tracking and stable multi-isotopes for estimating habitat use and winter range in Palearctic ospreys. Oecologia 195, 655–666 (2021). https://doi.org/10.1007/s00442-021-04855-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04855-5