Abstract
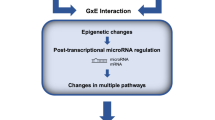
In the modern world, various forms of stressful conditions are an important problem for human health. The prolonged stress experienced by residents of large cities can lead to a decrease in cognitive functions and cause the development of anxiety disorders, depressive states, and more serious diseases. Studying the effect of stress and its consequences on the human body, as well as the development of antistress drugs, is an important task for modern science. The changes in the functioning of genes are the molecular genetic factors of stress manifestations; however, the mechanisms by which stress affects the functioning of genes are not fully understood. Analysis of the transcriptome underlies the study of gene functioning and is one of the most effective approaches to studying the mechanisms that determine the development of stress conditions and ways to achieve antistress effects of drugs. It has now been established that, in response to pathological effects, not only information RNAs (mRNAs) are involved, but also various types of noncoding RNAs, in particular, microRNAs and long noncoding RNAs (lncRNAs). Recently, the idea that lncRNAs can interact with microRNAs and inhibit their activity is actively being developed. Such functions are attributed to a new and actively studied type of RNA of circular nature (circRNAs). Recently, it has become apparent that the analysis of “noncoding RNA–mRNA” regulatory interactions is an important component part of the detailed study of the mechanisms of pathogenesis and stress-induced disorders. This review presents the latest data on the role of mRNAs and noncoding RNAs in acute stress, as well as under the action of antistress drugs.
Similar content being viewed by others
REFERENCES
Rüedi-Bettschen, D., Zhang, W., Russig, H., Ferger, B., Weston, A., Pedersen, E.M., et al., Early deprivation leads to altered behavioral, autonomic and endocrine responses to environmental challenge in adult Fischer rats, Eur. J. Neurosci., 2006, vol. 24, no. 10, pp. 2879–2893. https://doi.org/10.1111/j.1460-9568.2006.05158.x
Hasler, G., Drevets, W.C., Manji, H.K., and Charney, D.S., Discovering endophenotypes for major depression, Neuropsychopharmacology, 2004, vol. 29, no. 10, pp. 1765–1781. https://doi.org/10.1038/sj.npp.1300506
Jauregui-Huerta, F., Zhang, L., Yañez-Delgadillo, G., Hernandez-Carrillo, P., García-Estrada, J., and Luquín, S., Hippocampal cytogenesis and spatial learning in senile rats exposed to chronic variable stress: effects of previous early life exposure to mild stress, Front. Aging Neurosci., 2015, vol. 7, p. 159. https://doi.org/10.3389/fnagi.2015.00159
Shea, C.J., Carhuatanta, K.A., Wagner, J., Bechmann, N., Moore, R., Herman, J.P., et al., Variable impact of chronic stress on spatial learning and memory in BXD mice, Physiol. Behav., 2015, vol. 150, pp. 69–77. https://doi.org/10.1016/j.physbeh.2015.06.022
Schwabe, L., Memory under stress: From single systems to network changes, Eur. J. Neurosci., 2017, vol. 45, no. 4, pp. 478–489. https://doi.org/10.1111/ejn.13478
Bogdanov, M. and Schwabe, L., Transcranial stimulation of the dorsolateral prefrontal cortex prevents stress-induced working memory deficits, J. Neurosci., 2016, vol. 36, no. 4, pp. 1429–1437. https://doi.org/10.1523/JNEUROSCI.3687-15.2016
Cazakoff, B.N., Johnson, K.J., and Howland, J.G., Converging effects of acute stress on spatial and recognition memory in rodents: A review of recent behavioural and pharmacological findings, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2010, vol. 34, no. 5, pp. 733–741. https://doi.org/10.1016/j.pnpbp.2010.04.002
Li, S., Fan, Y.-X., Wang, W., and Tang, Y.-Y., Effects of acute restraint stress on different components of memory as assessed by object-recognition and object-location tasks in mice, Behav. Brain Res., 2012, vol. 227, no. 1, pp. 199–207. https://doi.org/10.1016/j.bbr.2011.10.007
Vargas-López, V., Lamprea, M.R., and Múnera, A., Histone deacetylase inhibition abolishes stress-induced spatial memory impairment, Neurobiol. Learn. Mem., 2016, vol. 134, part B, pp. 328–338. https://doi.org/10.1016/j.nlm.2016.08.009
Abrahám, I.M. and Kovács, K.J., Postnatal handling alters the activation of stress-related neuronal circuitries, Eur. J. Neurosci., 2000, vol. 12, no. 8, pp. 3003–3014.
Amin, S.N., Gamal, S.M., Esmail, R.S.E.N., Aziz, T.M.A., and Rashed, L.A., Cognitive effects of acute restraint stress in male albino rats and the impact of pretreatment with quetiapine versus ghrelin, J. Integr. Neurosci., 2014, vol. 13, no. 4, pp. 669–692. https://doi.org/10.1142/S0219635214500253
Haider, S., Naqvi, F., Batool, Z., Tabassum, S., Sadir, S., Liaquat, L., et al., Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats, Brain Res. Bull., 2015, vol. 115, pp. 1–8. https://doi.org/10.1016/j.brainresbull.2015.04.001
Glazova, N.Yu., Sebentsova, E.A., Manchenko, D.M., Andreeva, L.A., Dergunova, L.V., Levitskaya, N.G., et al., The protective effect of Semax in a model of stress-induced impairment of memory and behavior in white rats, Biol. Bull. (Moscow), 2018, vol. 45, no. 4, pp. 394–399. https://doi.org/10.1134/S1062359018040040
Ma, K., Guo, L., Xu, A., Cui, S., and Wang, J.-H., Molecular mechanism for stress-induced depression assessed by sequencing miRNA and mRNA in medial prefrontal cortex, PLoS One, 2016, vol. 11, no. 7, p. e0159093. https://doi.org/10.1371/journal.pone.0159093
Opmeer, E.M., Kortekaas, R., and Aleman, A., Depression and the role of genes involved in dopamine metabolism and signalling, Prog. Neurobiol., 2010, vol. 92, no. 2, pp. 112–133. https://doi.org/10.1016/j.pneurobio.2010.06.003
Whitton, A.E., Treadway, M.T., and Pizzagalli, D.A., Reward processing dysfunction in major depression, bipolar disorder and schizophrenia, Curr. Opin. Psychiatry, 2015, vol. 28, no. 1, pp. 7–12. https://doi.org/10.1097/YCO.0000000000000122
Zhou, M., Wang, M., Wang, X., Liu, K., Wan, Y., Li, M., et al., Abnormal expression of microRNAs induced by chronic unpredictable mild stress in rat hippocampal tissues, Mol. Neurobiol., 2018, vol. 55, no. 2, pp. 917–935. https://doi.org/10.1007/s12035-016-0365-6
Hendrickson, D.G., Hogan, D.J., McCullough, H.L., Myers, J.W., Herschlag, D., Ferrell, J.E., et al., Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA, PLoS Biol., 2009, vol. 7, no. 11, p. e1000238. https://doi.org/10.1371/journal.pbio.1000238
Wilczynska, A. and Bushell, M., The complexity of miRNA-mediated repression, Cell Death Differ., 2015, vol. 22, no. 1, pp. 22–33. https://doi.org/10.1038/cdd.2014.112
Liu, B.-B., Luo, L., Liu, X.-L., Geng, D., Liu, Q., and Yi, L.-T., 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: Through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress, Psychopharmacology (Berlin, Ger.), 2015, vol. 232, no. 3, pp 541–550. https://doi.org/10.1007/s00213-014-3690-3
Tay, Y., Kats, L., Salmena, L., Weiss, D., Tan, S.M., Ala, U., et al., Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs, Cell, 2011, vol. 147, no. 2, pp. 344–357. https://doi.org/10.1016/j.cell.2011.09.029
Broderick, J.A. and Zamore, P.D., Competitive endogenous RNAs cannot alter microRNA function in vivo, Mol. Cell, 2014, vol. 54, no. 5, pp. 711–713. https://doi.org/10.1016/j.molcel.2014.05.023
Denzler, R., Agarwal, V., Stefano, J., Bartel, D.P., and Stoffel, M., Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance, Mol. Cell, 2014, vol. 54, no. 5, pp. 766–776. https://doi.org/10.1016/j.molcel.2014.03.045
Salzman, J., Gawad, C., Wang, P.L., Lacayo, N., and Brown, P.O., Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types, PLoS One, 2012, vol. 7, no. 2, p. e30733. https://doi.org/10.1371/journal.pone.0030733
Jeck, W.R., Sorrentino, J.A., Wang, K., Slevin, M.K., Burd, C.E., Liu, J., et al., Circular RNAs are abundant, conserved, and associated with ALU repeats, RNA, 2013, vol. 19, no. 2, pp. 141–157. https://doi.org/10.1261/rna.035667.112
Zhang, Y., Zhang, X.O., Chen, T., Xiang, J.F., Yin, Q.F., Xing, Y.H., et al., Circular intronic long noncoding RNAs, Mol. Cell, 2013, vol. 51, no. 6, pp. 792–806. https://doi.org/10.1016/j.molcel.2013.08.017
Lasda, E. and Parker, R., Circular RNAs: Diversity of form and function, RNA, 2014, vol. 20, no. 12, pp. 1829–1842. https://doi.org/10.1261/rna.047126.114
Filippenkov, I.B., Kalinichenko, E.O., Limborska, S.A., and Dergunova, L.V., Circular RNAs—one of the enigmas of the brain, Neurogenetics, 2017, vol. 18, no. 1, pp. 1–6. https://doi.org/10.1007/s10048-016-0490-4
Filippenkov, I.B., Sudarkina, O.Yu., Limborska, S.A., and Dergunova, L.V., Circular RNA of the human sphingomyelin synthase 1 gene: Multiple splice variants, evolutionary conservatism and expression in different tissues, RNA Biol., 2015, vol. 12, no. 9, pp. 1030–1042. https://doi.org/10.1080/15476286.2015.1076611
Hansen, T.B., Jensen, T.I., Clausen, B.H., Bramsen, J.B., Finsen, B., Damgaard, C.K., et al., Natural RNA circles function as efficient microRNA sponges, Nature, 2013, vol. 495, no. 7441, pp. 384–388. https://doi.org/10.1038/nature11993
Lukiw, W.J., Circular RNA (circRNA) in Alzheimer’s disease (AD), Front. Genet., 2013, vol. 4, p. 307. https://doi.org/10.3389/fgene.2013.00307
Piwecka, M., Glažar, P., Hernandez-Miranda, L.R., Memczak, S., Wolf, S.A., Rybak-Wolf, A., et al., Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function, Science, 2017, vol. 357, no. 6357, p. eaam8526. https://doi.org/10.1126/science.aam8526
Zhang, H., Chen, Z., Zhong, Z., Gong, W., and Li, J., Total saponins from the leaves of Panax notoginseng inhibit depression on mouse chronic unpredictable mild stress model by regulating circRNA expression, Brain Behav., 2018, vol. 8, no. 11, p. e01127. https://doi.org/10.1002/brb3.1127
An, T., Zhang, J., Ma, Y., Lian, J., Wu, Y.X., Lv, B.H., et al., Relationships of non-coding RNA with diabetes and depression, Sci. Rep., 2019, vol. 9, no. 1, p. 10707. https://doi.org/10.1038/s41598-019-47077-9
Ashmarin, I.P., Samonina, G.E., Lyapina, L.A., Kamenskii, A.A., Levitskaya, N.G., Grivennikov, I.A., et al., Natural and hybrid chimeric stable regulatory glyproline peptides, Pathophysiology, 2005, vol. 11, no. 4, pp. 179–185. https://doi.org/10.1016/j.pathophys.2004.10.001
Kolomin, T.A., Agapova, T.Yu., Agniullin, Ya.V., Shram, S.I., Shadrina, M.I., Slominskii, P.A., et al., Transcriptome alteration in hippocampus under the treatment of tuftsin analog Selank, Zh. Vyssh. Nervn. Deyat. im. I.P. Pavlova, 2013, vol. 63, no. 3, pp. 365–374. https://doi.org/10.7868/s0044467713030052
Volkova, A., Shadrina, M., Kolomin, T., Andreeva, L., Limborska, S., Myasoedov, N., et al., Selank administration affects the expression of some genes involved in GABAergic neurotransmission, Front. Pharmacol., 2016, vol. 7, p. 31. https://doi.org/10.3389/fphar.2016.00031
An, T., He, Z.C., Zhang, X.Q., Li, J., Chen, A.L., Tan, F., et al., Baduanjin exerts anti-diabetic and anti-depression effects by regulating the expression of mRNA, lncRNA, and circRNA, Chin. Med., 2019, vol. 14, p. 3. https://doi.org/10.1186/s13020-019-0225-1
Funding
This work was supported by the Russian Science Foundation, project no. 19-14-00268.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Kazantseva
ADDITIONAL INFORMATION
Filippenkov I.B., https://orcid.org/0000-0002-6964-3405; e-mail: filippenkov@img.ras.ru.
Dergunova L.V., https://orcid.org/0000-0003-2789-2419; e-mail: lvdergunova@mail.ru.
About this article
Cite this article
Filippenkov, I.B., Dergunova, L.V. The Role of Coding and Regulatory RNAs during Acute Stress. Mol. Genet. Microbiol. Virol. 35, 129–133 (2020). https://doi.org/10.3103/S0891416820030027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416820030027