Abstract
This investigation reports the synthesis and evaluation of a novel and improved diatomite modified with Fe–Mn binary oxide (DT-FeMn) Fenton catalysis for photochemical reactions in aqueous media. The catalyst was prepared by a co-precipitation process and found to be highly efficient for the photocatalytic degradation Rhodamine B (RhB). In comparison with the iron trioxide coating on diatomite (DT-Fe), the nanoparticles on the surface of the DT-FeMn contained multiple active sites. Experimental results indicated that there was a synergy between the Fe and Mn in the Fe–Mn binary oxide, which was the most important factor for the improved catalytic oxidation performance. The stability and reusability of the composite coating of the catalyst were also investigated by comparing the photocatalytic performance and the loss of Fe3+ ions. It was found that the synergy between the components of the Fe–Mn binary oxide coating enhanced the stability of Fe3+ ions, which prevented the loss of the Fe3+ ions. A maximum of 99.1% RhB was degraded after 80 min. This work might provide a novel Fenton material for pollutants removal in the presence of H2O2 to alleviate environmental pollution.

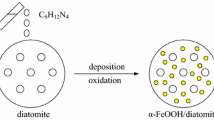
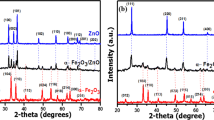
Diatomite-based DT-FeMn composites synthesized via one step co-precipitation and then structurally characterized. Under tungsten filament lamp irradiation, the hybrid materials showed much higher photo-Fenton catalytic activity for the RhB degradation than that of the single Fe2O3 coated, and a reasonable synergy mechanism between Fe2O3 and MnO2 was proposed.
Highlights
-
We synthesized circular pores of honeycomb structure hybrid material, Fe2O3 and MnO2 nanoparticles coated diatomite, which can effectively regulate the morphology of materials, and used to a heterogeneous catalyst for photo-Fenton degradation of organic contaminants.
-
The synergy between Fe2O3 and MnO2 enhanced the catalytic property (for 99.14% decoloration of organic dye) of diatomite-Fe2O3/MnO2 under visible light irradiation.
-
The novel diatomite-Fe2O3/MnO2 showed a new inroad to improve the photocatalytic performance of silicon-based Fenton materials, which was agreed with the journal requirements.
-
Diatomite was used to a novel silicon-based Fenton materials. The synergy between Fe2O3 and MnO2 enhanced the catalytic property, and improved the stability and reusability of the composite coating of the catalyst.









Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Banerjee S, Nigam V, Gautam RK, Halder S, Chattopadhyaya MC (2013) Removal of an anionic dye, Orange G from aqueous solutions by adsorption on unmodified sheesham (Dalbergia sisso) saw dust. J Indian Chem Soc 90:555–563
Khraisheh MAM, Al-Ghouti MA, Allen SJ, Ahmad MN (2005) Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res 39:922–932
Banat IM, Nigam P, Singh D, Marchant R (1996) Microbial decolorization of textile-dye-containing effluents: a review. Bioresour Technol 58(3):217–227
Potgieter JH, Pardesi C, Pearson S (2020) A kinetic and thermodynamic investigation into the removal of methyl orange from wastewater utilizing fly ash in different process configurations. Environ Geochem Health. https://doi.org/10.1007/s10653-020-00567-6
Wu Y, Li X, Yang Q, Wang D, Xu Q, Yao F, Chen F, Tao Z, Huang X (2019) Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J Environ Manage 231:370–379
Khataee A, Gholami P, Sheydaei M, Khorram S, Joo SW (2016) Preparation of nanostructured pyrite with N2 glow discharge plasma and study of its catalytic performance in heterogeneous fenton process. New J Chem 40(6):5221–5230
Hsien K, Tsai W, Su T (2009) Preparation of diatomite-TiO2 composite for photodegradation of bisphenol-A in water. J Sol-Gel Sci Technol 51:63–69
Guo S, Yang W, You LM, Li J, Chen JY, Zhou K (2020) Simultaneous reduction of Cr(VI) and degradation of tetracycline hydrochloride by a novel iron-modified rectorite composite through heterogeneous photo-Fenton processes. Chem Eng J 393:124758
Guo S, Wang HJ, Yang W, Fida H, You LM, Zhou K (2020) Scalable synthesis of Ca-doped alpha-Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl Catal B-Environ 262:118250
Chicinas RP, Gal E, Bedelean H, Darabantu M, Maicaneanu A (2018) Novel metal modified diatomite, zeolite and carbon xerogel catalysts for mild conditions wet air oxidation of phenol: characterization, efficiency and reaction pathway. Sep and Purif Technol 197:36–46
Yao GY, Lei JJ, Zhang XY, Sun ZM, Zheng SL (2018) One-step hydrothermal synthesis of zeolite X powder from natural low-grade diatomite. Materials 11(6):906
Du YC, Wang XK, Wu JS, Wang JS, Li Y, Dai HX (2018) Mg3Si4O10(OH)2 and MgFe2O4 in situ grown on diatomite: highly efficient adsorbents for the removal of Cr(VI). Microporous Mesoporous Mater 271:83–91
Li JQ, Xu J, Xie ZQ, Gao X, Zhou JY, Xiong Y, Chen CG, Zhang J, Liu ZF (2018) Diatomite-templated synthesis of freestanding 3D graphdiyne for energy storage and catalysis application. Adv Mater 30(20):1800548.1–1800548.8
Yan S, Huo WL, Yang JL, Zhang XY, Wang QG, Wang L, Pan YM, Huang Y (2018) Green synthesis and influence of calcined temperature on the formation of novel porous diatomite microspheres for efficient adsorption of dyes. Powder Technol 329:260–269
Zhu PF, Chen YJ, Duan M, Liu M, Zou P (2018) Structure and properties of Ag3PO4/diatomite photocatalysts for the degradation of organic dyes under visible light irradiation. Powder Technol 336:230–239
Wang B, Zhang GX, Leng X, Sun ZM, Zheng SL (2015) Characterization and improved solar light activity of vanadium doped TiO2/diatomite hybrid catalysts. J Hazard Mater 285:212–220
Meng X, Liu ZM, Deng C, Zhu MF, Wang DY, Li K, Deng Y, Jiang MM (2016) Microporous nano-MgO/diatomite ceramic membrane with high positive surface charge for tetracycline removal. J Hazard Mater 320:495–503
Shamsayei M, Yamini Y, Asiabi H (2020) A novel diatomite supported layered double hydroxide as reusable adsorbent for efficient removal of acidic dyes. Int J Environ Anal Chem 1:1–17
Chu HQ, Cao DW, Dong BZ, Qiang ZM (2010) Bio-diatomite dynamic membrane reactor for micro-polluted surface water treatment. Water Res 44(5):1573–1579
Knoerr R, Brendle J, Lebeau B, Demais H (2011) Elaboration of copper hydroxide phase modified diatomite and their application in lead ions immobilization. New J Chem 35(2):461–468
Deng LL, Du PX, Yu WB, Yuan P, Annabi-Bergaya F, Liu D, Zhou JM (2019) Novel hierarchically porous allophane/diatomite nanocomposite for benzene adsorption. Appl Clay Sci 168:155–163
Yuan P, Fan MD, Yang D, He HP, Liu D, Yuan AH, Zhu JX, Chen TH (2009) Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J Hazard Mater 166(2-3):821–829
Yuan P, Liu D, Fan MD, Yang D, Zhu RL, Ge F, Zhu JX, He HP (2019) Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J Hazard Mater 173(1-3):614–621
Liang H, Zhou S, Chen YT, Zhou F, Yan CJ (2015) Diatomite coated with Fe2O3 as an efficient heterogeneous catalyst for degradation of organic pollutant. J Taiwan Inst Chem Eng 49:105–112
Yu ZH, Zhang YF, Zhai SR, Wang Y, Pan YZ, Meng CG (2016) Amino-modified mesoporous sorbents for efficient Cd(II) adsorption prepared using non-chemical diatomite as precursor. J Sol-Gel Sci Technol 78:110–119
Jiang YL, Liu L, Xiao S, Chen JM (2016) Preparation of a novel manganese oxide-modified diatomite and its aniline removal mechanism from solution. Chem Eng J 284:609–619
Chen Y, Wu Q, Zhou C, Jin Q (2018) Facile preparation of Ce-doped TiO2/diatomite granular composite with enhanced photocatalytic activity. Adv Powder Technol 29(1):106–116
Ajenifuja E, Popoola API, Oyedotun KO, Popoola O (2018) Microstructural and porosimetry analysis of Ag-TiO2 intercalated kaolin and diatomite as nanocomposite ceramic materials. Clay Miner 53(4):665–674
Guo XL, Kuang M, Li F, Liu XY, Zhang YX, Dong F, Losic D (2016) Engineering of three dimensional (3-D) diatom@TiO2@MnO2 composites with enhanced supercapacitor performance. Electrochim Acta 190:159–167
Al-Degs Y, Khraisheh MAM, Tutunji MF (2001) Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res 35(15):3724–3728
He Y, Jiang B, Jiang Y, Chen J, Zhang YX (2018) Evaluation of MnO2-templated iron oxide-coated diatomites for their catalytic performance in heterogeneous photo Fenton-like system. J Hazard Mater 344:230–240
Mohamed A, Ghobara MM, Abdelmaksoud MK, Mohamed GG (2019) A novel and highly efficient photocatalytic degradation of malachite green dye via surface modified polyacrylonitrile nanofibers/biogenic silica composite nanofibers. Sep and Purif Technol 210:935–942
Dang TD, Banerjee AN, Cheney MA, Qian S, Joo SW, Min BK (2013) Bio-silica coated with amorphous manganese oxide as an efficient catalyst for rapid degradation of organic pollutant. Coll Surf B Biointerfaces 106:151–157
Xia P, Wang XJ, Wang X, Song JK, Wang H, Zhang J, Zhao JF (2016) Struvite crystallization combined adsorption of phosphate and ammonium from aqueous solutions by mesoporous MgO-loaded diatomite. Coll Surf A 506:220–227
Yuan P, He HP, Wu DQ, Wang DQ, Chen LJ (2004) Characterization of diatomaceous silica by Raman spectroscopy. Spectrochim Acta A 60(12):2941–2945
Yuan P, Wu DQ, He HP, Lin ZY (2004) The hydroxyl species and acid sites on diatomite surface: a combined IR and Raman study. Appl Surf Sci 227(1-4):30–39
Pang JB, Fu FL, Li WB, Zhu LJ, Tang B (2019) Fe-Mn binary oxide decorated diatomite for rapid decolorization of methylene blue with H2O2. Appl Surf Sci 478:54–61
Yuan P, Liu D, Tan DY, Liu KK, Yu HG, Zhong YH, Yuan AH, Yu WB, He HP (2013) Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu(II) adsorption: The effects of heating pretreatment. Microporous Mesoporous Mater 170:9–19
Zeng TY, Shi DJ, Cheng QR, Liao GY, Zhou H, Pan ZQ (2020) Construction of novel phosphonate-based MOF/P-TiO2 heterojunction photocatalysts: enhanced photocatalytic performance and mechanistic insight. Environ Sci-Nano 7(3):861–879
Funding
This work was supported by the Science and Technology Research Project of Education Department of Hubei Province (No. B2019054), and the Research Fund Program of the Key Laboratory of Rare Minerals, Ministry of Land and Resources (No. KLRM-KF201905), Hubei Technology Innovation Project (Foreign Science and Technology Cooperation; No. X19S012), and Hubei Tailings (Slag) Resource Utilization Engineering Technology Research Center Project (No. ZYYD201900072).
Author contributions
DD: data curation, investigation, writing—original draft; HL: conceptualization, ethodology, writing—review & editing, funding acquisition; DH: supervision, funding acquisition; HP: review & editing, alidation; ML: validation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dai, D., Liang, H., He, D. et al. Mn-doped Fe2O3/diatomite granular composite as an efficient Fenton catalyst for rapid degradation of an organic dye in solution. J Sol-Gel Sci Technol 97, 329–339 (2021). https://doi.org/10.1007/s10971-020-05452-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05452-3