Mesophilic Anaerobic Digestion of Hydrothermally Pretreated Lignocellulosic Biomass (Norway Spruce (Picea abies))
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hot Water Extraction (HWE) Producing Hydrolysates
2.2. Synthetic Hydrolysate
2.3. AD Batch Reactors Feeding
2.4. Methane Potential Test in AMPTS II
2.5. Methane Potential Test in Syringe
2.6. Analytical Methods
2.7. Kinetic Modeling
2.8. Statistical Analyses
3. Results
3.1. Characteristics of Hydrolysate
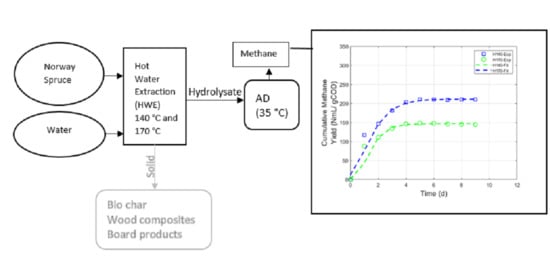
3.2. AMPTS II Test
3.3. Kinetic Modeling
3.4. Syringe Tests
3.4.1. OL Influence on AD of Hydrolysate
3.4.2. Methane Content and pH
4. Discussion
4.1. Effect of Sugars
4.2. Effect of Sugar Degradation Products
4.3. Effect of Soluble Lignin and Its Derivatives
4.4. Effect of OL
4.5. Kinetic Modeling
4.6. Comparison of AD Batch Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| AMPTS | Automatic Methane Potential Testing System |
| COD | Chemical Oxygen Demand |
| DM | Dry Matter |
| F/M | Feed to Microorganism Ratio |
| H140 | Hydrolysate Pretreated at 140 °C |
| H140syn | Synthetic feed simulating H140 |
| H170 | Hydrolysate Pretreated at 170 °C |
| H170syn | Synthetic feed simulating H170 |
| HMF | Hydroxymethyl Furfural |
| HWE | Hot Water Extraction |
| MMLD | Mini-Mill Laboratory Digester |
| OL | Organic Load |
| OLR | Organic Loading Rate |
| SS AD | Solid-State Anaerobic Digestion |
| VFA | Volatile Fatty Acid |
References
- Pelaez-Samaniego, M.R.; Yadama, V.; Lowell, E.; Espinoza-Herrera, R. A review of wood thermal pretreatments to improve wood composite properties. Wood Sci. Technol. 2013, 47, 1285–1319. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.J.; Cara, C.; Ruiz, E.; Pérez-Bonilla, M.; Castro, E. Hydrothermal pre-treatment and enzymatic hydrolysis of sunflower stalks. Fuel 2011, 90, 3225–3229. [Google Scholar] [CrossRef]
- Baeta, B.E.; Lima, D.R.; Adarme, O.F.; Gurgel, L.V.; Aquino, S.F. Optimization of sugarcane bagasse autohydrolysis for methane production from hemicellulose hydrolyzates in a biorefinery concept. Bioresour. Technol. 2016, 200, 137–146. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Chang, S.; Zhao, Z.; Zheng, A.; Li, X.; Wang, X.; Huang, Z.; He, F.; Li, H. Effect of hydrothermal pretreatment on properties of bio-oil produced from fast pyrolysis of eucalyptus wood in a fluidized bed reactor. Bioresour. Technol. 2013, 138, 321–328. [Google Scholar] [CrossRef]
- Alvarez-Chavez, B.J.; Godbout, S.; Palacios-Rios, J.H.; Le Roux, É.; Raghavan, V. Physical, chemical, thermal and biological pre-treatment technologies in fast pyrolysis to maximize bio-oil quality: A critical review. Biomass Bioenergy 2019, 128. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Le Roux, É.; Chaouch, M.; Diouf, P.N.; Stevanovic, T. Impact of a pressurized hot water treatment on the quality of bio-oil produced from aspen. Biomass Bioenergy 2015, 81, 202–209. [Google Scholar] [CrossRef]
- Torry-Smith, M.; Sommer, P.; Ahring, B.K. Purification of bioethanol effluent in an UASB reactor system with simultaneous biogas formation. Biotechnol. Bioeng. 2003, 84, 7–12. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Kongjan, P.; Sompong, O.T.; Kotay, M.; Min, B.; Angelidaki, I. Biohydrogen production from wheat straw hydrolysate by dark fermentation using extreme thermophilic mixed culture. Biotechnol. Bioeng. 2010, 105, 899–908. [Google Scholar] [CrossRef]
- Kádár, Z.; De Vrije, T.; Van Noorden, G.E.; Budde, M.A.; Szengyel, Z.; Réczey, K.; Claassen, P.A. Yields from glucose, xylose, and paper sludge hydrolysate during hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. In Proceedings of the 25th Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; pp. 497–508. [Google Scholar]
- Benjamin, M.M.; Woods, S.L.; Ferguson, J.F. Anaerobic toxicity and biodegradability of pulp mill waste constituents. Water Res. 1984, 18, 601–607. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.R.; Merlin Christy, P. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, J.; Zhang, J.; He, Y.; Zhang, R.; Chen, C.; Liu, G. Enhanced methane production of vinegar residue by response surface methodology (RSM). AMB Express 2017, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Li, Y.; Chen, C.; Liu, X.; Xiao, X.; Ma, X.; Zhang, R.; He, Y.; Liu, G. Biochemical methane potential (BMP) of vinegar residue and the influence of feed to inoculum ratios on biogas production. Bioresources 2013, 8, 2487–2498. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefin. 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Costa, A.G.; Pinheiro, G.C.; Pinheiro, F.G.C.; Dos Santos, A.B.; Santaella, S.T.; Leitão, R.C. The use of thermochemical pretreatments to improve the anaerobic biodegradability and biochemical methane potential of the sugarcane bagasse. Chem. Eng. J. 2014, 248, 363–372. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, N.; Bakke, R.; Bergland, W.H. Thermophilic Methane Production from Hydrothermally Pretreated Norway Spruce (Picea abies). Appl. Sci. 2020, 10, 4989. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Liu, S. A synergetic pretreatment technology for woody biomass conversion. Appl. Energy 2015, 144, 114–128. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Baeta, B.E.; Luna, H.J.; Sanson, A.L.; Silva, S.Q.; Aquino, S.F. Degradation of a model azo dye in submerged anaerobic membrane bioreactor (SAMBR) operated with powdered activated carbon (PAC). J. Environ. Manag. 2013, 128, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Badshah, M.; Lam, D.M.; Liu, J.; Mattiasson, B. Use of an Automatic Methane Potential Test System for evaluating the biomethane potential of sugarcane bagasse after different treatments. Bioresour. Technol. 2012, 114, 262–269. [Google Scholar] [CrossRef]
- Ostgaard, K.; Kowarz, V.; Shuai, W.; Henry, I.A.; Sposob, M.; Haugen, H.H.; Bakke, R. Syringe test screening of microbial gas production activity: Cases denitrification and biogas formation. J. Microbiol. Methods 2017, 132, 119–124. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Huang, H.; Zhang, Z.; Lei, Z.; Lin, B.-L. Energy Recovery from Rice Straw through Hydrothermal Pretreatment and Subsequent Biomethane Production. Energy Fuels 2017, 31, 10850–10857. [Google Scholar] [CrossRef]
- Ribeiro, F.R.; Passos, F.; Gurgel, L.V.A.; Baeta, B.E.L.; de Aquino, S.F. Anaerobic digestion of hemicellulose hydrolysate produced after hydrothermal pretreatment of sugarcane bagasse in UASB reactor. Sci. Total Environ. 2017, 584–585, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cegri, V.; Angeles De la Rubia, M.; Raposo, F.; Borja, R. Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quemeneur, M.; Trably, E.; Steyer, J.P.; Carrere, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lidén, G. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 87, 169–174. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Hydrothermal processing of lignocellulosic material. Holz Roh Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Barakat, A.; Monlau, F.; Steyer, J.P.; Carrere, H. Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour. Technol. 2012, 104, 90–99. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Jimenez, S.; Cartagena, M.C.; Arce, A. Influence of lignin on the methanization of lignocellulosic wastes. Biomass 1990, 21, 43–54. [Google Scholar] [CrossRef]
- Benner, R.; Maccubbin, A.; Hodson, R.E. Anaerobic biodegradation of the lignin and polysaccharide components of lignocellulose and synthetic lignin by sediment microflora. Appl. Environ. Microbiol. 1984, 47, 998–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, A.; Gaillard, C.; Steyer, J.-P.; Carrere, H. Anaerobic Biodegradation of Cellulose–Xylan–Lignin Nanocomposites as Model Assemblies of Lignocellulosic Biomass. Waste Biomass Valoriz. 2013, 5, 293–304. [Google Scholar] [CrossRef]
- Kleinheinz, G.; Hernandez, J. Comparison of two laboratory methods for the determination of biomethane potential of organic feedstocks. J. Microbiol. Methods 2016, 130, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P. Biogas Measurement Techniques and the Associated Errors; University of Jyväskylä: Jyväskylä, Finland, 2011. [Google Scholar]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S. Identification of Critical Problems in Biochemical Methane Potential (BMP) Tests from Methane Production Curves. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]






| Parameters | H140syn | H170syn |
|---|---|---|
| Soluble COD (g CODs/L) | 12.6 | 20.7 |
| Arabinose (g/L) | 1.63 | 0.81 |
| Galactose (g/L) | 1.67 | 2.17 |
| Glucose (g/L) | 1.55 | 3.00 |
| Xylose (g/L) | 1.95 | 2.24 |
| Mannose (g/L) | 5.11 | 10.39 |
| Acetic acid (g/L) | 0.59 | 1.03 |
| pH | 3.14 | 3.07 |
| Sample | Inoculum (mL) | Substrate (mL) | OL (g CODt/L) | Parallels |
|---|---|---|---|---|
| H140 | 200 | 200 | 20 | 3 |
| H170 | 240 | 160 | 20 | 3 |
| H140syn | 160 | 240 | 20 | 3 |
| H170syn | 200 | 200 | 20 | 3 |
| Control (Blank) | 240 | 160 (DW) | - | 3 |
| Sample | Inoculum (mL) | Substrate (mL) | OL (g CODt/L) | Parallels |
|---|---|---|---|---|
| H140 | 15 | 4 | 6 | 3 |
| H140 | 15 | 6.7 | 10 | 3 |
| H140 | 15 | 13.4 | 20 | 3 |
| H140 | 15 | 20 | 30 | 3 |
| H170 | 15 | 3 | 6 | 3 |
| H170 | 15 | 5 | 10 | 3 |
| H170 | 15 | 10 | 20 | 3 |
| H170 | 15 | 15 | 30 | 3 |
| Control (Blank) | 15 | 10 (DW) | - | 3 |
| Samples | Initial pH | End pH | Initial CODs mg/L (Feed Only) | End CODs mg/L (Feed and Inoculum) | End Acetic Acid (mg/L) | End Propionic Acid (mg/L) | End Total VFA (mg/L) | Methane Yield (g COD/g COD) |
|---|---|---|---|---|---|---|---|---|
| H140 | 6.1 | 6.9 ± 0.0 | 20,000 | 1933 ± 63 | ND | ND | ND | 0.60 ± 0.02 |
| H170 | 5.9 | 7.0 ± 0.1 | 20,000 | 1723 ± 62 | ND | ND | ND | 0.41 ± 0.00 |
| H140syn | 6.1 | 7.6 ± 0.1 | 20,000 | 1670 ± 216 | ND | ND | ND | 0.74 ± 0.01 |
| H170syn | 6.0 | 7.7 ± 0.1 | 20,000 | 2313 ± 68 | ND | ND | ND | 0.72 ± 0.02 |
| Samples | G0 (NmL CH4/g COD) | Rmax (NmL CH4/(g COD d)) | λ (d) | R2 | Cumulative Methane Yield (NmL/g COD) |
|---|---|---|---|---|---|
| H140 | 211 | 79 | 0.04 | 0.958 | 210 |
| H170 | 146 | 78 | 0.44 | 0.931 | 148 |
| H140syn | 288 | 13 | 1.27 | 0.860 | 260 |
| H170syn | 284 | 12 | 1.99 | 0.863 | 252 |
| OL (g COD/L) | Weighted Average H140 | Final H140 | Weighted Average H170 | Final H170 |
|---|---|---|---|---|
| 6 | 63.6 ± 0.8 | 83.9 ± 0.6 | 64.7 ± 0.5 | 85.4 ± 0.7 |
| 10 | 57.8 ± 5.5 | 80.0 ± 6.9 | 63.8 ± 3.8 | 84.6 ± 7.5 |
| 20 | 64.5 ± 1.1 | 80.1 ± 5.4 | 76.5 ± 1 | 82.2 ± 2.1 |
| 30 | 65.3 ± 1.7 | 80.5 ± 2.4 | 72.9 ± 1.0 | 76.1 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, N.; Bakke, R.; Bergland, W.H. Mesophilic Anaerobic Digestion of Hydrothermally Pretreated Lignocellulosic Biomass (Norway Spruce (Picea abies)). Processes 2021, 9, 190. https://doi.org/10.3390/pr9020190
Ghimire N, Bakke R, Bergland WH. Mesophilic Anaerobic Digestion of Hydrothermally Pretreated Lignocellulosic Biomass (Norway Spruce (Picea abies)). Processes. 2021; 9(2):190. https://doi.org/10.3390/pr9020190
Chicago/Turabian StyleGhimire, Nirmal, Rune Bakke, and Wenche Hennie Bergland. 2021. "Mesophilic Anaerobic Digestion of Hydrothermally Pretreated Lignocellulosic Biomass (Norway Spruce (Picea abies))" Processes 9, no. 2: 190. https://doi.org/10.3390/pr9020190