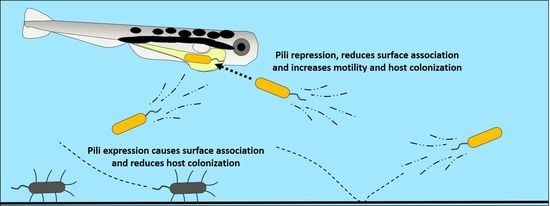
Msh Pilus Mutations Increase the Ability of a Free-Living Bacterium to Colonize a Piscine Host
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Comparative Analysis Suggests MshL-T300P Is a Loss-of-Function Mutation
3.2. MshL-T300P Behaves Similarly to a MshL Deletion Mutant
3.3. Evolved Lineages Show No Improvements in Carrying Capacity In Vivo
3.4. L3a and T300P Have Similar In Vivo Growth Rates to the wt MR-1 Strain
3.5. L3a and T300P Outcompete the wt MR-1 Strain in Larvae-Conditioned Medium
3.6. L3a and MshL-T300P Increase per Capita Immigration into the Gut
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachs, J.L.; Skophammer, R.G.; Regus, J.U. Evolutionary transitions in bacterial symbiosis. Proc. Natl. Acad. Sci. USA 2011, 108, 10800–10807. [Google Scholar] [CrossRef] [Green Version]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.M.; Mittge, E.; Kuhlman, J.; Baden, K.N.; Cheesman, S.E.; Guillemin, K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 2006, 297, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef]
- Lebov, J.F.; Schlomann, B.H.; Robinson, C.D.; Bohannan, B.J.M. Phenotypic Parallelism during Experimental Adaptation of a Free-Living Bacterium to the Zebrafish Gut. bioRxiv 2020, 11. [Google Scholar] [CrossRef]
- McKee, R.W.; Aleksanyan, N.; Garrett, E.M.; Tamayo, R. Type IV Pili Promote Clostridium difficile Adherence and Persistence in a Mouse Model of Infection. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; Li, S.-H.; Yang, Y.-C.; Hsu, S.-H.; Nizet, V.; Chang, Y.-C. T4 Pili Promote Colonization and Immune Evasion Phenotypes of Nonencapsulated M4 Streptococcus pyogenes. mBio 2020, 11. [Google Scholar] [CrossRef]
- Spaulding, C.N.; Schreiber, H.L., IV; Zheng, W.; Dodson, K.W.; Hazen, J.E.; Conover, M.S.; Wang, F.; Svenmarker, P.; Luna-Rico, A.; Francetic, O.; et al. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. eLife 2018, 7, e31662. [Google Scholar] [CrossRef] [Green Version]
- Ligthart, K.; Belzer, C.; De Vos, W.M.; Tytgat, H.L. Bridging Bacteria and the Gut: Functional Aspects of Type IV Pili. Trends Microbiol. 2020, 28, 340–348. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.; Houeix, B.; Kilcoyne, M.; Joshi, L.; Boyd, A. The MSHA pilus of Vibrio parahaemolyticus has lectin functionality and enables TTSS-mediated pathogenicity. Int. J. Med Microbiol. 2013, 303, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.; Liu, Z.; Joelsson, A.; Zhu, J. Vibrio cholerae virulence regulator-coordinated evasion of host immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 14542–14547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saville, R.M.; Dieckmann, N.; Spormann, A.M. Spatiotemporal activity of the mshA gene system in Shewanella oneidensis MR-1 biofilms. FEMS Microbiol. Lett. 2010, 308, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.J.; Utada, A.S.; Davis, K.R.; Thongsomboon, W.; Sanchez, D.Z.; Banakar, V.; Cegelski, L.; Wong, G.C.L.; Yildiz, F.H. C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in Vibrio cholerae. PLOS Pathog. 2015, 11, e1005068. [Google Scholar] [CrossRef]
- Bonazzi, D.; Schiavo, V.L.; Machata, S.; Djafer-Cherif, I.; Nivoit, P.; Manriquez, V.; Tanimoto, H.; Husson, J.; Henry, N.; Chaté, H.; et al. Intermittent Pili-Mediated Forces Fluidize Neisseria meningitidis Aggregates Promoting Vascular Colonization. Cell 2018, 174, 143–155.e6. [Google Scholar] [CrossRef]
- Bordeleau, E.; Purcell, E.B.; Lafontaine, D.A.; Fortier, L.-C.; Tamayo, R.; Burrus, V. Cyclic Di-GMP Riboswitch-Regulated Type IV Pili Contribute to Aggregation of Clostridium difficile. J. Bacteriol. 2015, 197, 819–832. [Google Scholar] [CrossRef] [Green Version]
- Maldarelli, G.A.; Piepenbrink, K.H.; Scott, A.J.; Freiberg, J.A.; Song, Y.; Achermann, Y.; Ernst, R.K.; Shirtliff, M.E.; Sundberg, E.J.; Donnenberg, M.S.; et al. Type IV pili promote early biofilm formation byClostridium difficile. Pathog. Dis. 2016, 74, ftw061. [Google Scholar] [CrossRef] [Green Version]
- Sivadon, P.; Barnier, C.; Urios, L.; Grimaud, R. Biofilm formation as a microbial strategy to assimilate particulate substrates. Environ. Microbiol. Rep. 2019, 11, 749–764. [Google Scholar] [CrossRef]
- Ardita, C.S.; Mercante, J.W.; Kwon, Y.M.; Luo, L.; Crawford, M.E.; Powell, D.N.; Jones, R.M.; Neish, A.S. Epithelial Adhesion Mediated by Pilin SpaC Is Required for Lactobacillus rhamnosus GG-Induced Cellular Responses. Appl. Environ. Microbiol. 2014, 80, 5068–5077. [Google Scholar] [CrossRef] [Green Version]
- Mazariego-Espinosa, K.; Cruz, A.; Ledesma, M.A.; Ochoa, S.A.; Xicohtencatl-Cortes, J. Longus, a Type IV Pilus of Enterotoxigenic Escherichia coli, Is Involved in Adherence to Intestinal Epithelial Cells. J. Bacteriol. 2010, 192, 2791–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tytgat, H.L.P.; Nobrega, F.L.; van der Oost, J.; de Vos, W.M. Bowel Biofilms: Tipping Points between a Healthy and Compromised Gut? Trends Microbiol. 2019, 27, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, E.S.; Bittinger, K.; Esipova, T.V.; Hou, L.; Chau, L.; Jiang, J.; Mesaros, C.; Lund, P.J.; Liang, X.; Fitzgerald, G.A.; et al. Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc. Natl. Acad. Sci. USA 2018, 115, 4170–4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Ochoa, V.E.; Lam, D.; Lee, C.S.; Klaus, S.; Behnsen, J.; Liu, J.Z.; Chim, N.; Nuccio, S.-P.; Rathi, S.G.; Mastroianni, J.R.; et al. Salmonella Mitigates Oxidative Stress and Thrives in the Inflamed Gut by Evading Calprotectin-Mediated Manganese Sequestration. Cell Host Microbe 2016, 19, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Genet. 2016, 14, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Stoodley, P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005, 13, 7–10. [Google Scholar] [CrossRef]
- Vuong, C.; Voyich, J.M.; Fischer, E.R.; Braughton, K.R.; Whitney, A.R.; DeLeo, F.R.; Otto, M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 2004, 6, 269–275. [Google Scholar] [CrossRef]
- Melancon, E.; Canny, S.G.D.L.T.; Sichel, S.; Kelly, M.; Wiles, T.; Rawls, J.; Eisen, J.; Guillemin, K. Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods Cell Biol. 2017, 138, 61–100. [Google Scholar]
- Milligan-Myhre, K.; Charette, J.R.; Phennicie, R.T.; Stephens, W.Z.; Rawls, J.F.; Guillemin, K.; Kim, C.H. Study of host–microbe interactions in zebrafish. Methods Cell Biol. 2011, 105, 87–116. [Google Scholar]
- Wiles, T.J.; Wall, E.S.; Schlomann, B.H.; Hay, E.A.; Parthasarathy, R.; Guillemin, K. Modernized Tools for Streamlined Genetic Manipulation and Comparative Study of Wild and Diverse Proteobacterial Lineages. mBio 2018, 9, e01877-18. [Google Scholar] [CrossRef] [Green Version]
- Deatherage, D.E.; Barrick, J.E. Identification of Mutations in Laboratory-Evolved Microbes from Next-Generation Sequencing Data Using breseq. Methods Mol. Biol. 2014, 1151, 165–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenski, R.E.; Rose, M.R.; Simpson, S.C.; Tadler, S.C. Long-Term Experimental Evolution in Escherichia coli. I. Adaptation and Divergence During 2000 Generations. Am. Nat. 1991, 138, 1315–1341. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Thormann, K.M.; Saville, R.M.; Shukla, S.; Pelletier, D.A.; Spormann, A.M. Initial Phases of Biofilm Formation in Shewanella oneidensis MR-1. J. Bacteriol. 2004, 186, 8096–8104. [Google Scholar] [CrossRef] [Green Version]
- Howard, S.P.; Estrozi, L.F.; Bertrand, Q.; Contreras-Martel, C.; Strozen, T.; Job, V.; Martins, A.; Fenel, D.; Schoehn, G.; Dessen, A. Structure and assembly of pilotin-dependent and -independent secretins of the type II secretion system. PLoS Pathog. 2019, 15, e1007731. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, L.A.; Petersen, E.R.; Ray, R.I.; Little, B.J.; Cooper, C.J.; Howard, E.C.; Ringeisen, B.R.; Biffinger, J.C. Shewanella oneidensis MR-1 Msh pilin proteins are involved in extracellular electron transfer in microbial fuel cells. Process. Biochem. 2012, 47, 170–174. [Google Scholar] [CrossRef]
- Denise, R.; Abby, S.S.; Rocha, E.P. Diversification of the type IV filament superfamily into machines for adhesion, protein secretion, DNA uptake, and motility. PLoS Biol. 2019, 17, e3000390. [Google Scholar] [CrossRef] [Green Version]
- Chou, P.Y.; Fasman, G.D. Prediction of protein conformation. Biochemistry 1974, 13, 222–245. [Google Scholar] [CrossRef]
- Savojardo, C.; Fariselli, P.; Martelli, P.L.; Casadio, R. INPS-MD: A web server to predict stability of protein variants from sequence and structure. Bioinformatics 2016, 32, 2542–2544. [Google Scholar] [CrossRef]
- Monk, I.; Casey, P.G.; Cronin, M.; Gahan, C.G.M.; Hill, C. Development of multiple strain competitive index assays for Listeria monocytogenes using pIMC; a new site-specific integrative vector. BMC Microbiol. 2008, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebov, J.F. Experimental Adaptation of a Free-Living Bacterium to the Zebrafish Digestive Tract. Ph.D. Thesis, University of Oregon, Eugene, OR, USA, 2019. [Google Scholar]
- Jemielita, M.; Taormina, M.J.; Burns, A.R.; Hampton, J.S.; Rolig, A.S.; Guillemin, K.; Parthasarathy, R. Spatial and Temporal Features of the Growth of a Bacterial Species Colonizing the Zebrafish Gut. mBio 2014, 5, e01751-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, J.G.; Zamorano-Sánchez, D.; Park, J.H.; Sondermann, H.; Yildiz, F.H. The ins and outs of cyclic di-GMP signaling in Vibrio cholerae. Curr. Opin. Microbiol. 2017, 36, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariyakumar, D.S.; Nishiguchi, M.K. Characterization of two host-specific genes, mannose-sensitive hemagglutinin (mshA) and uridyl phosphate dehydrogenase (UDPDH) that are involved in the Vibrio fischeri–Euprymna tasmanica mutualism. FEMS Microbiol. Lett. 2009, 299, 65–73. [Google Scholar] [CrossRef]
- List, C.; Grutsch, A.; Radler, C.; Cakar, F.; Zingl, F.G.; Schild-Prüfert, K.; Schild, S. Genes Activated by Vibrio cholerae upon Exposure to Caenorhabditis elegans Reveal the Mannose-Sensitive Hemagglutinin To Be Essential for Colonization. Msphere 2018, 3, e00238-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hottes, A.K.; Freddolino, P.L.; Khare, A.; Donnell, Z.N.; Liu, J.C.; Tavazoie, S. Bacterial Adaptation through Loss of Function. PLoS Genet. 2013, 9, e1003617. [Google Scholar] [CrossRef]
- Barrick, J.E.; Lenski, R.E. Genome dynamics during experimental evolution. Nat. Rev. Genet. 2013, 14, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Batista, J.; Sousa, A.; Lourenço, M.; Bergman, M.-L.; Sobral, D.; Demengeot, J.; Xavier, K.B.; Gordo, I. The First Steps of Adaptation of Escherichia coli to the Gut Are Dominated by Soft Sweeps. PLoS Genet. 2014, 10, e1004182. [Google Scholar] [CrossRef]
- Cont, A.; Rossy, T.; Al-Mayyah, Z.; Persat, A. Biofilms deform soft surfaces and disrupt epithelia. eLife 2020, 9. [Google Scholar] [CrossRef]
- Frese, S.A.; MacKenzie, D.A.; Peterson, D.A.; Schmaltz, R.; Fangman, T.; Zhou, Y.; Zhang, C.; Benson, A.K.; Cody, L.A.; Mulholland, F.; et al. Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont. PLoS Genet. 2013, 9, e1004057. [Google Scholar] [CrossRef]
- Blanchette, K.A.; Orihuela, C.J. Future perspective on host–pathogen interactions during bacterial biofilm formation within the nasopharynx. Future Microbiol. 2012, 7, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.J.; Shivshankar, P.; Stol, K.; Trakhtenbroit, S.; Sullam, P.M.; Sauer, K.; Hermans, P.W.M.; Orihuela, C.J. The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation In Vivo and in Biofilms. PLoS Pathog. 2010, 6, e1001044. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Raina, J.-B.; Fernandez, V.; Lambert, B.; Stocker, R.; Seymour, J.R. The role of microbial motility and chemotaxis in symbiosis. Nat. Rev. Genet. 2019, 17, 284–294. [Google Scholar] [CrossRef]
- Kim, Y.-C. Diffusivity of bacteria. Korean J. Chem. Eng. 1996, 13, 282–287. [Google Scholar] [CrossRef]
- Robinson, C.D.; Klein, H.S.; Murphy, K.D.; Parthasarathy, R.; Guillemin, K.; Bohannan, B.J.M. Experimental bacterial adaptation to the zebrafish gut reveals a primary role for immigration. PLoS Biol. 2018, 16, e2006893. [Google Scholar] [CrossRef] [Green Version]
- Stephens, W.Z.; Wiles, T.J.; Martinez, E.S.; Jemielita, M.; Burns, A.R.; Parthasarathy, R.; Bohannan, B.J.M.; Guillemin, K. Identification of Population Bottlenecks and Colonization Factors during Assembly of Bacterial Communities within the Zebrafish Intestine. mBio 2015, 6, e01163-15. [Google Scholar] [CrossRef] [Green Version]
- Schlomann, B.H.; Wiles, T.J.; Wall, E.S.; Guillemin, K.; Parthasarathy, R. Bacterial Cohesion Predicts Spatial Distribution in the Larval Zebrafish Intestine. Biophys. J. 2018, 115, 2271–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiles, T.J.; Jemielita, M.; Baker, R.P.; Schlomann, B.H.; Logan, S.L.; Ganz, J.; Melancon, E.; Eisen, J.S.; Guillemin, K.; Parthasarathy, R. Host Gut Motility Promotes Competitive Exclusion within a Model Intestinal Microbiota. PLoS Biol. 2016, 14, e1002517. [Google Scholar] [CrossRef] [PubMed]
- Wiles, T.J.; Schlomann, B.H.; Wall, E.S.; Betancourt, R.; Parthasarathy, R.; Guillemin, K. Swimming motility of a gut bacterial symbiont promotes resistance to intestinal expulsion and enhances inflammation. PLoS Biol. 2020, 18, e3000661. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebov, J.F.; Bohannan, B.J.M. Msh Pilus Mutations Increase the Ability of a Free-Living Bacterium to Colonize a Piscine Host. Genes 2021, 12, 127. https://doi.org/10.3390/genes12020127
Lebov JF, Bohannan BJM. Msh Pilus Mutations Increase the Ability of a Free-Living Bacterium to Colonize a Piscine Host. Genes. 2021; 12(2):127. https://doi.org/10.3390/genes12020127
Chicago/Turabian StyleLebov, Jarrett F., and Brendan J. M. Bohannan. 2021. "Msh Pilus Mutations Increase the Ability of a Free-Living Bacterium to Colonize a Piscine Host" Genes 12, no. 2: 127. https://doi.org/10.3390/genes12020127