Functionalization of Betulinic Acid with Polyphenolic Fragments for the Development of New Amphiphilic Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Remarks
2.1.2. Synthesis of Methyl Betulonate (7)
2.1.3. Synthesis of Methyl (E)-2-Benzylidenebetulonate Derivatives 9a–d and 14, and Methyl (E,E)-2-[-3-(3,4-Dimethoxyphenyl)allylidene]betulonate (12)
2.1.4. Synthesis of 19,28-Epoxyoleanane-3,28-dione-Derived Polyhydroxylated Compounds 4a–d and 5
2.1.5. Synthesis of Methyl (E)-2-(3,4-Dihydroxybenzylidene)betulonate (6)
2.2. DPPH• and ABTS•+ Scavenging Assays
3. Results and Discussion
3.1. Synthesis of Polyhydroxylated (E)-2-Benzylidene-19,28-epoxyoleanane-3,28-diones 4a–d
3.2. Radical Scavenging Activity of Polyhydroxylated (E)-2-Benzylidene-19,28-epoxyoleanane-3,28-diones 4a–d
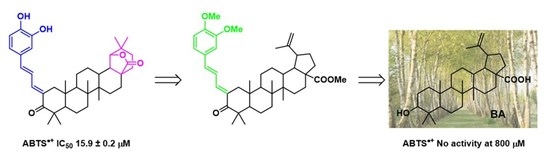
3.3. Analogues Synthesis of the Hit Compound 4c and Evaluation of Their Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghaddam, M.G.; Ahmad, F.B.H.; Samzadeh-Kermani, A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regueiro-Ren, A.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.-Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Zhu, J.; Nowicka-Sans, B.; et al. Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity. ACS Med. Chem. Lett. 2016, 7, 568–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.E.; Salzwedel, K.; Allaway, G.P. Bevirimat: A Novel Maturation Inhibitor for the Treatment of HIV-1 Infection. Antiviral Chem. Chemother. 2008, 19, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Xu, H.-G.; Wang, L.; Li, Y.-J.; Sun, P.-H.; Wu, X.-M.; Wang, G.-J.; Chen, W.-M.; Ye, W.-C. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Patents 2014, 24, 913–923. [Google Scholar] [CrossRef]
- Gupta, N.; Rath, S.K.; Singh, J.; Qayum, A.; Singh, S.; Sangwan, P.L. Synthesis of novel benzylidene analogues of betulinic acid as potent cytotoxic agents. Eur. J. Med. Chem. 2017, 135, 517–530. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Cheng, M.-L.; Chiang, M.-C.; Chen, C.-M. Lipophilic antioxidants in neurodegenerative diseases. Clin. Chim. Acta 2018, 485, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chong-Han, K. Dietary Lipophilic Antioxidants: Implications and Significance in the Aging Process. Crit. Rev. Food Sci. Nutr. 2010, 50, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Lagouri, V. Lipophilic Antioxidants. In Lipids and Skin Health; Pappas, A., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 301–310. [Google Scholar]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorg. Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.P.R.; Silva, A.M.S.; Seixas, R.S.G.R.; Pinto, D.C.G.A.; Tomé, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef]
- Gomes, A.; Freitas, M.; Fernandes, E.; Lima, J.L.F.C. Biological Activities of 2-Styrylchromones. Mini-Rev. Med. Chem. 2010, 10, 1–7. [Google Scholar] [CrossRef]
- Komissarova, N.G.; Belenkova, N.G.; Shitikova, O.V.; Spirikhin, L.V.; Yunusov, M.S. Cyclopropanation of betulonic acid and its methyl ester with dichlorocarbene generated under phase transfer catalysis conditions. Russ. Chem. Bull. 2005, 54, 2659–2663. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arabian J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum L. decoctions. Food Funct. 2017, 8, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, W.; Mashentseva, A.A.; Seitembetov, T.S. Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules 2011, 16, 2443–2466. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Porto, G.; Fernandes, E. Modulation of human neutrophils’ oxidative burst by flavonoids. Eur. J. Med. Chem. 2013, 67, 280–292. [Google Scholar] [CrossRef] [PubMed]









| Compound | R | IC50 (µM) | |
|---|---|---|---|
| DPPH• | ABTS•+ | ||
| Precursors | |||
| BA (1) | — | NA800 μM | NA800 μM |
| 7 | — | NA800 μM | NA800 μM |
| Compounds 4a–d (Section 3.1) | |||
| 4a | H | NA800 μM | NA800 μM |
| 4b | 4-OH | NA800 μM | NA800 μM |
| 4c | 3,4-(OH)2 | 22.1 ± 0.6 | 29.8 ± 0.1 |
| 4d | 3,4,5-(OH)3 | 27.5 ± 0.4 | 35.1 ± 0.3 |
| Hit 4c analogues (Section 3.3) | |||
| 5 | — | 24.6 ± 0.6 | 15.9 ± 0.2 |
| 6 | — | 25.1 ± 0.1 | 19.1 ± 0.3 |
| Positive controls | |||
| α-Tocopherol | — | 24.0 ± 0.4 | 19.2 ± 0.1 |
| Quercetin | — | 11.4 ± 0.9 | 5.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, J.L.C.; Gonçalves, C.; Ferreira, R.M.; Cardoso, S.M.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Functionalization of Betulinic Acid with Polyphenolic Fragments for the Development of New Amphiphilic Antioxidants. Antioxidants 2021, 10, 148. https://doi.org/10.3390/antiox10020148
Sousa JLC, Gonçalves C, Ferreira RM, Cardoso SM, Freire CSR, Silvestre AJD, Silva AMS. Functionalization of Betulinic Acid with Polyphenolic Fragments for the Development of New Amphiphilic Antioxidants. Antioxidants. 2021; 10(2):148. https://doi.org/10.3390/antiox10020148
Chicago/Turabian StyleSousa, Joana L. C., Cristiana Gonçalves, Ricardo M. Ferreira, Susana M. Cardoso, Carmen S. R. Freire, Armando J. D. Silvestre, and Artur M. S. Silva. 2021. "Functionalization of Betulinic Acid with Polyphenolic Fragments for the Development of New Amphiphilic Antioxidants" Antioxidants 10, no. 2: 148. https://doi.org/10.3390/antiox10020148