Abstract
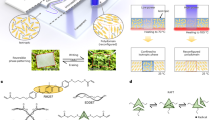
Surface modification of versatile polymeric materials without changing their bulk properties is one of the essential techniques for regulating their physical and chemical characteristics, and this technique can improve the functions of materials. In this study, a bottlebrush-type poly[oligo(2-ethyl-2-oxazoline) methacrylate] (P[O(Ox)nMA]) (n = 7 and 19) was synthesized as a surface modifier. This compound was mixed into poly(methyl methacrylate) (PMMA) as a matrix (PMMA/P[O(Ox)nMA]) with a weight ratio of 15%, and the aggregation state in the surface region was examined under air and aqueous environments via atomic force microscopy, contact angle measurement, angular-dependent X-ray photoelectron spectroscopy, and neutron reflectivity. The surface of the PMMA/P[O(Ox)nMA] films was flat at the subnanometer level and covered with the PMMA-rich phase. However, once the films contacted water, the surface was reorganized due to the migration of P[O(Ox)nMA]. The extent of the surface segregation was more remarkable for P[O(Ox)7MA] than P[O(Ox)19MA] due to the entropic factor. Concurrently, NIH3T3 fibroblast adhesion and serum protein adsorption on the film were more strongly suppressed for P[O(Ox)7MA] than P[O(Ox)19MA] because it formed a thicker diffused interface in the film with water.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Pinson J, Thiry D Surface modification of polymers: methods and applications. Weinheim, Germany: Wiley-VCH Verlag; 2019.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–30.
Yang C, Ding X, Ono RJ, Lee H, Hsu LY, Tong YW, et al. Brush‒like polycarbonates containing dopamine, cations, and PEG providing a broad‒spectrum, antibacterial, and antifouling surface via one‒step coating. Adv Mater. 2014;26:7346–51.
Park CH, Lee SY, Hwang DS, Shin DW, Cho DH, Lee KH, et al. Nanocrack-regulated self-humidifying membranes. Nature. 2016;532:480–3.
Tung S, Fisher SL, Kotov NA, Thompson LT. Nanoporous aramid nanofibre separators for nonaqueous redox flow batteries. Nat Commun. 2018;9:4193.
Eickenscheidt M, Singler E, Stieglitz T. Pulsed electropolymerization of PEDOT enabling controlled branching. Polym J. 2019;51:1029–36.
Tibbitt MW, Rodell CB, Burdick JA, Anseth KS. Progress in material design for biomedical applications. Proc Natl Acad Sci USA. 2015;112:14444–51.
Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, et al. Protein interactions with polymer coatings and biomaterials. Angew Chem Int Ed. 2014;53:8004–31.
Coessens V, Pintauer T, Matyjaszewski K. Functional polymers by atom transfer radical polymerization. Prog Polym Sci. 2001;26:337–77.
Tsujii Y, Ohno K, Yamamoto S, Goto A, Fukuda T Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization. In: Jordan R editor. Surface-initiated polymerization I, Adv Polym Sci. Berlin Heidelberg: Springer-Verlag; 2006. Vol. 197, p. 1–45.
Zoppe JO, Ataman NC, Mocny P, Wang J, Moraes J, Klok HA. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem Rev. 2017;117:1105–318.
Shiomoto S, Yamaguchi Y, Yamaguchi K, Nogata Y, Kobayashi M. Adhesion force measurement of live cypris tentacles by scanning probe microscopy in seawater. Polym J. 2019;51:51–9.
Zhang N, Pompe T, Amin I, Luxenhofer R, Werner C, Jordan R. Tailored poly(2‒oxazoline) polymer brushes to control protein adsorption and cell adhesion. Macromol Biosci. 2012;12:926–36.
Tang P, Sd Cio, Wang W, Gautrot JE. Surface-initiated poly(oligo(2-alkyl-2-oxazoline)methacrylate) brushes. Langmuir. 2018;34:10019–27.
Ishihara K, Yanokuchi S, Fukazawa K, Inoue Y. Photoinduced self-initiated graft polymerization of methacrylate monomers on poly(ether ether ketone) substrates and surface parameters for controlling cell adhesion. Polym J. 2020;52:731–41.
Zdyrko B, Luzinov I. Polymer brushes by the “grafting to” method. Macromol Rapid Commun. 2011;32:859–69.
Bai L, Tan L, Chen L, Liu S, Wang Y. Preparation and characterizations of poly(2-methyl-2-oxazoline) based antifouling coating by thermally induced immobilization. J Mater Chem B. 2014;2:7785–94.
Zheng X, Zhang C, Bai L, Liu S, Tan L, Wang Y. Antifouling property of monothiol-terminated bottle-brush poly(methylacrylic acid)-graft-poly(2-methyl-2-oxazoline) copolymer on gold surfaces. J Mater Chem B. 2015;3:1921–30.
Wang H, Li L, Tong Q, Yan M. Evaluation of photochemically immobilized poly(2-ethyl-2-oxazoline) thin films as protein-resistant surfaces. ACS Appl Mater Interfaces. 2011;3:3463–71.
Hara M, Kitahata S, Nishimori K, Miyahara K, Morita K, Tokuda K, et al. Surface-functionalization of isotactic polypropylene via dip-coating with a methacrylate-based terpolymer containing perfluoroalkyl groups and poly(ethylene glycol). Polym J. 2019;51:489–99.
Bhatia QS, Pan DH, Koberstein JT. Preferential surface adsorption in miscible blends of polystyrene and poly(vinyl methyl ether). Macromolecules. 1988;21:2166–75.
Tanaka K, Kawaguchi D, Yokoe Y, Kajiyama T, Takahara A, Tasaki S. Surface segregation of chain ends in α,ω-fluoroalkyl-terminated polystyrenes films. Polymer. 2003;44:4171–7.
Oda Y, Inutsuka M, Awane R, Totani M, Yamada LN, Haraguchi M, et al. Dynamic interface based on segregation of an amphiphilic hyperbranched polymer containing fluoroalkyl and oligo(ethylene oxide) moieties. Macromolecules. 2020;53:2380–7.
Yamamoto K, Kawaguchi D, Abe T, Komino T, Mamada M, Kabe T, et al. Surface segregation of a star-shaped polyhedral oligomeric silsesquioxane in a polymer matrix. Langmuir. 2020;36:9960–6.
Tanaka K, Kajiyama T, Takahara A, Tasaki S. A novel method to examine surface composition in mixtures of chemically identical two polymers with different molecular weights. Macromolecules. 2002;35:4702–6.
Atarashi H, Ariura F, Akabori K, Ozawa M, Tanaka K, Nagamura T. Interfacial segregation of hyper-branched polystyrene in mixtures of linear component. Trans Mater Res Soc Jpn. 2007;32:231–4.
Hirai T, Liu H, Ohta Y, Yokozawa T, Tanaka K. Surface segregation of well-defied N-substituted hyperbranched polyamides in linear polymer matrix. Chem Lett. 2011;40:366–7.
Sugimoto S, Oda Y, Hirata T, Matsuyama R, Matsuno H, Tanaka K. Surface segregation of a branched polymer with hydrophilic poly[2-(2-ethoxy)ethoxyethyl vinyl ether] side chains. Polym Chem. 2017;8:505–10.
Matsuno H, Tsukamoto R, Oda Y, Tanaka K. Platelet adhesion on the surface of a simple poly(vinyl ether). Polymer. 2017;116:479–86.
Hirata T, Matsuno H, Kawaguchi D, Hirai T, Yamada NL, Tanaka M, et al. Effect of local chain dynamics on a bioinert interface. Langmuir. 2015;31:3661–7.
Inutsuka M, Ito K, Yamada NL, Yokoyama H. High density polymer brush spontaneously formed by the segregation of amphiphilic diblock copolymers to polymer/water interface. ACS Macro Lett. 2013;2:265–8.
Matsuno H, Tsukamoto R, Shimomura S, Hirai T, Oda Y, Tanaka K. Platelet-adhesion behavior synchronized with surface rearrangement in a film of poly(methyl methacrylate) terminated with elemental blocks. Polym J. 2016;48:413–9.
Tateishi Y, Kai N, Noguchi H, Uosaki K, Nagamura T, Tanaka K. Local conformation of poly(methyl methacrylate) at nitrogen and water interfaces. Polym Chem. 2010;1:303–11.
Hong JH, Totani M, Kawaguchi D, Masunaga H, Yamada NL, Matsuno H, et al. Design of a bioinert interface using an amphiphilic block copolymer containing a bottlebrush unit of oligo(oxazoline). ACS Appl Bio Mater. 2020;3:7363–8.
Matsuno H, Totani M, Yamamoto A, Haraguchi M, Ozawa M, Tanaka K. Water-induced surface reorganization of bioscaffolds composed of an amphiphilic hyperbranched polymer. Polym J. 2019;51:1045–53.
Shundo A, Hori K, Ikeda T, Kimizuka N, Tanaka K. Design of a dynamic polymer interface for chiral discrimination. J Am Chem Soc. 2013;135:10282–5.
Oda Y, Horinouchi A, Kawaguchi D, Matsuno H, Kanaoka S, Aoshima S, et al. Effect of side-chain carbonyl groups on the interface of vinyl polymers with water. Langmuir. 2014;30:1215–9.
Li X, ShamsiJazeyi H, Pesek SL, Agrawal A, Hammoud B, Verduzco R. Thermoresponsive PNIPAAM bottlebrush polymers with tailored side-chain length and end-group structure. Soft Matter. 2014;10:2008–15.
Verduzco R, Li X, Peseka SL, Stein GE. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem Soc Rev. 2015;44:2405–20.
Mitra I, Li X, Pesek SL, Makarenko B, Lokitz BS, Uhrig D, et al. Thin film phase behavior of bottlebrush/linear polymer blends. Macromolecules. 2014;47:5269–5276.
Oda Y. Construction of hydrophilic surfaces with poly(vinyl ether)s and their interfacial properties in water. Polym J. 2019;51:955–62.
Totani M, Liu L, Matsuno H, Tanaka K. Design of a star-like hyperbranched polymer having hydrophilic arms for anti-biofouling coating. J Mater Chem B. 2019;7:1045–9.
Gaertner FC, Luxenhofer R, Blechert B, Jordan R. Essler M. Synthesis, biodistribution and excretion of radiolabeled poly(2-alkyl-2-oxazoline)s. J Control Release. 2007;119:291–300.
Adams N, Schubert US. Poly(2-oxazolines) in biological and biomedical application contexts. Adv Drug Deliv Rev. 2007;59:1504–20.
Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K, Dizman B, et al. Polyoxazoline: Chemistry, properties, and applications in drug delivery. Bioconjug Chem. 2011;22:976–86.
Tauhardt L, Kempe K, Gottschaldt M, Schubert US. Poly(2-oxazoline) functionalized surfaces: From modification to application. Chem Soc Rev. 2013;42:7998–8011.
Jana S, Uchman M. Poly(2-oxazoline)-based stimulus-responsive (co)polymers: An overview of their design, solution properties, surface-chemistries and application. Prog Polym Sci. 2020;106:101252.
Lorson T, Lübtow MM, Wegener E, Haider MS, Borova S, Nahm D, et al. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials. 2018;178:204–80.
Weber C, Remzi Becer C, Guenther W, Hoogenboom R, Schubert US. Dual responsive methacrylic acid and oligo(2-ethyl-2-oxazoline) containing graft copolymers. Macromolecules. 2010;43:160–7.
Gieseler D, Jordan R. Poly(2-oxazoline) molecular brushes by grafting through of poly(2-oxazoline)methacrylates with aqueous ATRP. Polym Chem. 2015;6:4678–89.
Tsai TY, Huang CF. Data in support of dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Data Brief. 2015;3:195–200.
Pape PG Coupling agents, silanes (Adhesion Promoters). In: The polymeric materials encyclopedia. Salomone JC editor, Boca Raton, FL, US: CRC Press, 1996.
Yamada NL, Torikai N, Mitamura K, Sagehashi H, Sato S, Seto H, et al. Design and performance of horizontal-type neutron reflectometer SOFIA at J-PARC/MLF. Eur Phys J. 2011;126:108.
Mitamura K, Yamada NL, Sagehashi H, Torikai N, Arita H, Terada M, et al. Novel neutron reflectometer SOFIA at J-PARC/MLF for in-situ soft-interface characterization. Polym J. 2013;45:100–8.
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85.
Owens DK, Wendt RC. Estimation of the surface free energy of polymers. J Appl Polym Sci. 1969;13:1741–7.
Schneider HA. Conformational entropy contributions to the glass temperature of blends of miscible polymers. J Res Natl Inst Stand Technol. 1997;102:229–48.
Utracki LA Polymer Blends Handbook; Dordrecht, The Netherlands, and Boston, MA, US: Kluwer Academic Publishers, 2003.
Tanaka K, Fujii Y, Atarashi H, Akabori K, Hino M, Nagamura T. Nonsolvents cause swelling at the interface with poly(methyl methacrylate) films. Langmuir. 2008;24:296–301.
Acknowledgements
We are grateful for support from the JST-Mirai Program (JPMJMI18A2) (KT), JSPS KAKENHI Grant-in-Aids for Scientific Research (B) (JP20H02790) (KT) and (B) (JP18H02037) (HM), and for Early-Career Scientists (JP18K16990) (MT). The NR measurements were approved by the Neutron Scattering Program Advisory Committee of IMSS, KEK with Proposal Nos. 2018B0287, 2019A0255, 2019B0269, 2019B0365, 2020A0272, and 2017L2501.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, JH., Totani, M., Kawaguchi, D. et al. Poly[oligo(2-ethyl-2-oxazoline) methacrylate] as a surface modifier for bioinertness. Polym J 53, 643–653 (2021). https://doi.org/10.1038/s41428-020-00459-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00459-7
This article is cited by
-
Surface zeta potential and protein adsorption on the coating surface of a heteroarm star polymer with a controlled hydrophilic/hydrophobic arm ratio
Polymer Journal (2024)
-
Near-ambient pressure X-ray photoelectron spectroscopy for a bioinert polymer film at a water interface
Polymer Journal (2021)
-
Thermal hysteresis of aggregation states of thermoresponsive block copolymers forming intermolecular hydrogen bonds
Polymer Journal (2021)