Abstract
Disruption of the intestinal epithelial barrier following spinal cord injury (SCI) seriously affect long-term quality of life. Oxidative stress-induced epithelial cells’ injury contributes to the epithelial barrier dysfunction. Hyperbaric oxygen (HBO) treatment has been proved to alleviate SCI. However, it is unclear whether or not HBO treatment affects intestinal barrier function following SCI. In this study, our purpose was to explore the impact of HBO treatment on intestinal epithelial barrier function and underlying mechanisms following SCI. An SCI model was established in rats, and the rats received HBO treatment. Intestinal injury, mucosal permeability, intercellular junction proteins, and oxidative stress indicators were evaluated in our study. We found that HBO treatment significantly alleviated intestinal histological damage, reduced mucosal permeability, and markedly prevented bacterial translocation. Furthermore, HBO treatment significantly increased the expression of Claudin-1 and E-cadherin, inhibited intestinal tissue oxidative stress as demonstrated by upregulation of superoxide dismutase and glutathione, and HBO downregulated malondialdehyde. Mechanically, we demonstrated that HBO treatment ameliorated intestinal oxidative stress possibly through upregulating nuclear factor E2-related factor 2 (Nrf2) and its downstream targets, Heme oxygenase-1(HO-1), NADH-quinone oxidoreductase-1(NQO-1), and glutamate cysteine ligase catalytic subunit (GCLC). These results suggested that HBO treatment triggered antioxidative effects against intestinal epithelial barrier dysfunction by promoting Nrf2 signaling pathway after SCI.
Similar content being viewed by others
Introduction
Gastrointestinal disturbance is a common clinical complication following spinal cord injury (SCI) and compromises the long-term quality of life of individuals with SCI (Hou and Rabchevsky 2014). SCI initiated a series of intestinal pathophysiological changes such as ischemia of the intestinal mucosa, intestinal inflammation, increased intestinal permeability, and damage to intestinal epithelial barrier function (Besecker et al. 2017; O'Connor et al. 2018). Destroying or overwhelming the intestinal epithelial barrier facilitates translocation of luminal bacteria and antigens into the blood circulation and mesenteric lymph. Systemic inflammatory response syndrome (SIRS) is induced followed by multiple organ dysfunction syndrome (MODS) with the risk of high disability and fatality (Wu et al. 2017). Therefore, maintaining the intestinal barrier function is critical for the prognosis of individuals with SCI.
Major trauma-induced ischemia or hypoperfusion of the intestinal mucosa could promote a mass production of reactive oxygen species (ROS) which results in intestinal oxidative stress (Jin et al. 2008). Different signaling pathways could be activated by increased oxidative stress, which leads to the necrosis of intestinal enterocytes and then to the destruction of the intestinal epithelial barrier (Liu et al. 2017). Nuclear factor erythroid 2-related factor 2 (Nrf2) plays a crucial role in redox-sensitive reactions. Upon activation by oxidative stress, Nrf2 could coordinate with the antioxidant responsive element and preserve cells from oxidant insults (Bellezza et al. 2018).
As a well-established physical treatment, hyperbaric oxygen (HBO) treatment is the inhalation of 100% O2 exposed to more than one atmosphere absolute (Thom 2011). It has been widely demonstrated that HBO treatment can alleviate spinal cord injury and improve neurological function (Sun et al. 2019; Ying et al. 2019). However, no studies have reported the effect of HBO treatment on intestinal barrier function following SCI. HBO treatment increases dissolved oxygen in the blood, repairs the hypoxic tissue, and improves perfusion. A previous study found that HBO induced the production of H2O2 and strengthened antioxidant defense elements to prevent oxidative insults (Ferrer et al. 2007). Further, Godman et al. (2010) demonstrated that HBO treatment promoted the expression of Nrf2 in endothelial cells, indicating a cytoprotective effect via initiating an antioxidant response. Thus, we assumed that HBO treatment could improve intestinal barrier dysfunction after SCI.
In this research, our purpose was to study the impact of HBO treatment on intestinal epithelial barrier function following SCI. To clarify the molecular mechanisms involved, we focused on the changes in the Nrf2 signaling pathway after HBO treatment.
Materials and methods
Animals
Sprague-Dawley rats (male, aged 8–10 weeks, 250–300 g, n=36) purchased from the Experimental Animals Center of Capital Medical University were fed in a climate-stable animal room (22–24 °C; relative humidity, 50–60%) with enough food and water. After 1 week of acclimation, the rats were randomly assigned into three experimental groups (n=12 each group): sham-operated group (Sham), SCI group, and SCI + HBO group. All animals in this study were specific pathogen free (SPF), and the animal study was conducted with the approval from the Committee for Experimental Animal Welfare and Ethics, Beijing Chaoyang Hospital, Capital Medical University.
Animal model of SCI
Rats received a moderately severe spinal cord contusion by the MASCIS (Multicenter Animal Spinal Cord Injury Study) weight-drop device (Basso et al. 1996). Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (350 mg/kg) and were performed a laminectomy at level T10 with exposure of dura mater. The spinal cord (T10) suffered a fall injury with a 10.0 g rod dropping from a height of 25.0 mm. Afterwards, the muscles and skin were sutured carefully. Rats were given intensive care in a warmed cage (about 30 °C) to recover from anesthetic and operation with adequate water and food for 12 h. Bladders were compressed manually twice daily until the spontaneous urination reflex was restored. Rats in the Sham group underwent all surgical operations to expose the spinal cord but were not injured. All experimental rats were injected with penicillin (0.8 mg/g) once a day to prevent infections.
HBO treatment
Rats in the SCI+HBO group were treated with HBO treatment in a transparent animal hyperbaric oxygen chamber (701 research institute, Wuhan, China) 6 h after the operation as described previously (Liu et al. 2015). Rats were exposed to 100% O2 at 2.0 atm absolute (ATA) for 1 h once per day for 3 days consecutively. Compression and decompression were conducted at the rate of 0.2 ATA/min. To avoid the overproduction of CO2, the chamber was washed by pure O2 for 5 min before treatment. The temperature in the chamber was controlled at 22–25 °C. Rats in the Sham and SCI groups were placed in normobaric room air.
Histopathological assessment of intestines
On postoperative day 3, the rats were deeply anesthetized using chloral hydrate, and about 2 cm length of middle jejunum was obtained. One-half of the jejunums were fixed in 10% formalin for tissue section preparation, the other half of the jejunums were kept in − 80 °C refrigerator for intestinal tissue immunoblotting and real-time PCR.
We immobilized the jejunum segments in 10% formalin and embedded them in paraffin. These jejunum segments were cut into 5-μm sections for hematoxylin and eosin (H&E). The intestinal histopathological assessment was undertaken blindly by a pathologist under optical microscope based on Chiu’s method (Chiu et al. 1970). The Chiu’s method evaluates intestinal villi, subepithelial space, capillary congestion, separation of mucosa and lamina, hemorrhage, and disintegration of lamina propria with scores ranging from 0 (normal intestinal villi) to 5 (loss of intestinal villi, hemorrhage, and necrosis of lamina propria).
Assessment of intestinal permeability and bacterial translocation
On postoperative day 3, after fasting for 4 h, the rats were gavaged with 0.6 mg/g fluorescein isothiocyanate (FITC)-dextran (FD-40, 40 kD, Sigma) and then anesthetized after 4 h. The blood was then collected via cardiac puncture. The level of FITC in serum was measured using a spectrofluorophotometer.
Mesenteric lymph nodes (MLN) were excised aseptically, and 0.1 g was taken from each node for homogenization treatment. Diluted homogenates of 100 μl were cultured on MacConkey’s agar (Sigma-Aldrich, St Louis, MO, USA) for 24 h at 37 °C. In addition, blood and peritoneal fluid (PF) were also taken and diluted for bacterial colony counts lying on LB agar (Sigma-Aldrich, St Louis, MO, USA) for 24 h at 37 °C. To analyze the growth of bacteria on the agars, colony-forming units/g of tissue were calculated.
Biochemical detection
Diamine oxidase (DAO), D-lactate (D-lac), and intestinal fatty acid binding protein (I-FABP) in the serum were detected to assess intestinal barrier function. The activity of DAO is closely related to the nucleic acid and protein synthesis of mucosal cells and can reflect the integrity of the intestinal mechanical barrier and the degree of damage. D-lactate is a metabolite produced by bacterial fermentation, and its level can reflect the damage degree and permeability changes of intestinal mucosa. I-FABP only exists in the gastrointestinal mucosa and has a good correlation with the degree of intestinal ischemia injury. Malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) in intestinal tissue were detected to reflect the state of oxidative stress. All the above indicators were tested by corresponding reagent kits (DAO, A088-2-1; D-lac, H263; I-FABP, H265-1-1; MDA, A003-1-2; SOD, A001-1-2; GSH, A006-1-1, all from Nanjing Jiancheng, China) following the manufacturer’s instructions.
Immunohistochemical analysis
We deparaffinized intestinal sections using xylene three times and rehydrated them using ethanol and water. Then the antigens were extracted by microwave heating in EDTA buffer (pH 8.0). After blocking the activity of endogenous peroxidase, the sections were incubated with primary antibodies Claudin-1 (1:200, ab15098) and E-cadherin (1:500, ab15148) (all from Abcam, Cambridge, MA, USA) overnight. Horseradish peroxidase (HRP)-binded goat anti- rabbit IgG (Santa Cruz Biotechnology, Inc. USA) was added and incubated for 50 min. DAB chromogenic liquid was added to the sections and counterstained with hematoxylin. The presence of brownish yellow granules was considered positive protein expression. The average optical density (AOD) of positive staining in 5 fields per section and 5 sections per animal (n=6 per group) was analyzed using Image-Pro Plus 6.0 (Media Cybernetics, USA) software by a pathologist blindly under a high-times optical microscope.
Western blotting analysis
Frozen intestinal tissue was homogenized and total protein was quantified using the bicinchoninic acid (BCA) protein quantitative kit (Sun Bio, Beijing, China) following the manufacturer’s instructions. The extracted protein specimens (50 μg) from the intestinal tissue were segregated by electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membrane. After blocking, the PVDF membranes were incubated with primary antibodies to Claudin-1 (1:200, ab15098), E-cadherin (1:500, ab15148), Nrf2 (1:500, ab137550), heme oxygenase 1 (HO-1) (1:500, ab13243), NADPH-quinone oxidoreductase-1 (NQO1) (1:1000, ab217302), glutamate cysteine ligase catalytic subunit (GCLC) (1:1000, ab53179), and β-actin (1:2000) (all from Abcam, Cambridge, MA, USA) at 4 °C overnight. After washing three times, the membranes were incubated with the secondary antibodies IgG (Santa Cruz Biotechnology, Inc. USA) for 2 h at room temperature. The western blot bands were treated with an enhanced chemiluminescence (ECL) solution (Millipore Corporation, Billerica, MA, USA). The intensity of the protein bands was analyzed using Lab Works (UVP, Upland, CA, USA) software.
Real-time polymerase chain reaction
In accordance with the manufacturer’s instructions, we extracted the total RNA from the above intestinal tissue by Trizol reagent (Invitrogen, California, USA) and RNA kit (Biomed, Beijing, China). Then the extracted RNA was reverse transcribed into cDNA, and quantitative RT-PCR was conducted with the BioEasy SYBR Green I Real-Time PCR Kit and Line-Gene sequence detector (Bioer, Hangzhou, China). The primers for Nrf2, HO-1, NQO1, GCLC, and actin were listed in Table 1. PCR amplification was performed as follows: 95 °C for 2 min, 60 °C for 25 s, and 72 °C for 30 s. The Line-Gene software was used to analyze the data. The expression level of mRNA was calculated by the 2−ΔΔCT method.
Statistical analysis
The SPSS 19.0 (IBM Inc., Chicago, IL, USA) software was used to analyze the results, and the data were expressed as mean ± SD. We analyzed the differences between groups by one-way analysis of variance (ANOVA), and we regarded P < 0.05 as statistically significant.
Results
HBO treatment alleviates intestinal injury following SCI
As shown in Fig. 1a, the intestinal histological changes were examined by HE staining. Rats in the Sham group showed normal intestinal histomorphology. SCI rats exhibited damaged intestinal villi, capillary congestion, and increased crypt depth, while HBO treatment significantly preserved the integrity of intestinal morphological structures compared to the SCI group (P < 0.01). Simultaneously, Chiu’s scores of intestinal injury were markedly increased after SCI, but were reduced by HBO treatment.
HBO treatment ameliorates intestinal injury following SCI. (a) Representative images of intestinal histopathology (HE) and intestinal injury score; some of the key features in HE images were labeled using different colored arrows (black arrow, intestinal villi; red arrow, capillary; yellow arrow, crypt). (b) Analysis of DAO, D-lac, and I-FABP expression levels in serum. Data are expressed as mean ± SD. **P < 0.01 vs. Sham group; #P < 0.05, ##P < 0.01 vs. SCI group. DAO, diamine oxidase; D-lac, D-lactate; I-FABP, intestinal fatty acid binding protein
In addition, to characterize the intestinal lesion induced by SCI, the levels of DAO, D-lac, and I-FABP in serum were assessed as biomarkers of damage to the intestinal epithelium. The expression levels of DAO, D-lac, and I-FABP were significantly higher in the SCI and SCI + HBO groups than those of in the Sham group (P < 0.01), while HBO treatment significantly lowered the upregulation of these biomarkers compared to the SCI group (P < 0.05, P < 0.01, Fig. 1b). Taken together, these findings illustrated that HBO treatment protected intestinal mucosal against injury after SCI.
HBO treatment improves intestinal permeability and inhibits bacterial translocation following SCI
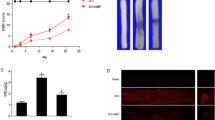
Compared to the Sham group, SCI increased intestinal permeability, which was proved by higher level of serum FD-40 (P < 0.01), while the serum FD-40 level was significantly lower in the SCI + HBO group than in that of the SCI group (P < 0.01, Fig. 2a), which showed that HBO treatment reduced the intestinal hyperpermeability after SCI.
HBO treatment improves intestinal permeability and prevents bacterial translocation following SCI. (a) Analysis of FD-40 level in serum; (b) culture results of bacterial growth in MLN, blood, and PF. Greater than 102 colonies/g of tissue were considered positive. Data are expressed as mean ± SD.**P < 0.01 vs. Sham group; ##P < 0.01 vs. SCI group. MLN, mesenteric lymph nodes; PF, peritoneal fluid
The damage to the intestinal barrier function leads to bacterial translocation from the intestinal cavity to the MLN, blood, and PF. As shown in Fig. 2b, SCI significantly promoted the translocation of bacteria from the intestinal cavity to the MLN, blood, and PF (P < 0.01). However, translocation of bacteria in the rats treated with HBO was inhibited compared to the untreated SCI rats (P < 0.01).
HBO treatment increases the expression of intestinal intercellular junctions following SCI
To estimate the influence of HBO treatment on intercellular junctions, we detected the expression of Claudin-1 and E-cadherin in intestinal tissue by immunohistochemistry and western blotting. As shown in Fig. 3a, SCI rats showed lower immunoactivity of Claudin-1 and E-cadherin in intestinal tissue compared to rats in the Sham group (P < 0.01), whereas the immunoactivity of these factors was significantly enhanced by HBO treatment (P < 0.01). Furthermore, western blotting analysis illustrated that the concentration of Claudin-1 and E-cadherin was significantly decreased after SCI (P < 0.01); however, the protein expression levels of these two factors were elevated in the SCI + HBO groups (P < 0.05, P < 0.01, Fig. 3b). These data indicated that HBO treatment increased the expression of intestinal intercellular junctions after SCI.
HBO treatment increases the expression of intestinal intercellular junctions following SCI. (a) Representative images of Claudin-1 and E-cadherin expression in intestinal tissue (immunohistochemistry) and analysis of the average optical density of positive staining; (b) representative immunoblot of Claudin-1 and E-cadherin (western blotting) and analysis of 2 factor protein expression. Data are expressed as mean ± SD.**P < 0.01 vs. Sham group; #P < 0.05,##P < 0.01 vs. SCI group
HBO treatment alleviates intestinal oxidative stress following SCI
As shown in Fig. 4, SCI induced an increase of MDA levels in intestinal tissue compared to the Sham group (P < 0.01), while HBO treatment markedly decreased MDA levels (P < 0.05). To evaluate enzyme activity, we detected the expression levels of intestinal SOD and GSH, and their antioxidant activities were significantly reduced following SCI (P < 0.01). However, HBO treatment significantly enhanced their antioxidant activities (P < 0.05).
HBO treatment alleviates intestinal oxidative stress through Nrf2 signaling following SCI
To explore the influence of HBO treatment on Nrf2 signaling, we detected the levels of Nrf2, HO-1, NQO-1, and GCLC in intestinal tissue using western blotting and RT-PCR analysis. Figure 5a showed that the concentrations of Nrf2-related antioxidant proteins were significantly higher in the SCI and SCI + HBO groups than those of the Sham group (P < 0.01). Relative to the SCI group, HBO treatment further significantly promoted the expression of these antioxidant proteins (P < 0.01). Similarly to the western blotting results, RT-PCR results also demonstrated that HBO treatment increased the expression of these Nrf2-related antioxidant genes compared with those in the SCI group (P < 0.01, Fig. 5b). These findings demonstrated that Nrf2 signaling might be involved in the protection against intestinal tissue oxidative stress by HBO treatment after SCI.
Effect of HBO treatment on Nrf2 signaling following SCI. (a) Representative immunoblot of Nrf2, HO-1, NQO-1, and GCLC in intestinal tissue (western blotting) and analysis of their protein expression; (b) analysis of mRNA levels of these 4 factors (RT-PCR). Data are expressed as mean ± SD. **P < 0.01 vs. Sham group; ##P < 0.01 vs. SCI group. Nrf2, nuclear factor E2-related factor 2; HO-1, heme oxygenase-1; NQO-1, NADH-quinone oxidoreductase-1; GCLC, glutamate cysteine ligase catalytic subunit
Discussion
In this research, we demonstrated that HBO treatment ameliorated intestinal mucosal injury after SCI, which showed that HBO treatment increased the expression of intercellular junction proteins, alleviated intestinal oxidative stress, maintained intestinal epithelial barrier function, and reduced bacteria translocation. Furthermore, we found that the protective mechanism of HBO was accompanied by upregulating some antioxidant genes, such as Nrf2, HO-1, NQO-1, and GCLC. These outcomes suggested that HBO treatment improved SCI-induced intestinal barrier function partly through activating the Nrf2 signaling pathway.
As a noninvasive and safe form of physical therapy, HBO treatment has been generally applied to the treatment of some injuries, soft tissue infections, and osteomyelitis, particularly for gas gangrene, acute CO poisoning, and decompression sickness (Ding et al. 2014). However, there are few reports on the study of HBO treatment for intestinal injury. Sakoda et al. (2004) reported that HBO treatment alleviates intestinal injury and maintains intestinal barrier function by activating the NF-κB signaling pathway in a sepsis rat model. In the ischemia-reperfusion (IR) rat model, HBO treatment reduced small intestinal IR injury by raising oxygen supply in blood, maintaining ATP and aerobic metabolism, and inhibiting inflammation and intestinal epithelium apoptosis (Zhou et al. 2012). In our study, we found SCI significantly induced intestinal mucosal injury and elevated the expression levels of DAO, D-lac, and I-FABP. Nevertheless, HBO treatment notably prevented these changes, which indicated that HBO treatment protected intestinal barrier function.
The intestinal epithelial barrier consists of simple intestinal epithelial cells and intercellular junction proteins of enterocytes. Intercellular junctions play a key role in stabilizing the function of the intestinal epithelial barrier (Tong et al. 2016). Novak et al. (2016) found that HBO treatment could activate genes encoding for tight junction proteins to stabilize the intestinal barrier in colitis mice. Consistently, in this research, we detected the expression of Claudin-1 and E-cadherin, which are important intercellular junction proteins. We found the expressions of Claudin-1 and E-cadherin were markedly reduced after SCI, while HBO treatment significantly prevented SCI-induced downregulation of Claudin-1 and E-cadherin. These results suggested that HBO treatment protected intestinal barrier function by upregulating intercellular junction proteins.
Ischemia or hypoperfusion of the intestinal mucosa following SCI broke down the balance between the oxidants and antioxidants leading to the occurrence of oxidative stress. Some researchers thought oxidative stress induces intestinal enterocytes’ apoptosis and therefore contributes to damaged intestinal mucosal barrier function (Williams et al. 2013). Oxidative stress induced the activation of antioxidant enzymes (SOD, GSH, etc.) which play a critical role in eliminating ROS and lipid peroxidation products (primarily MDA) (Zhang et al. 2019). There is evidence that HBO pretreatment induced ischemic tolerance by upregulating the activity of antioxidant enzymes (Xue et al. 2016; Xu et al. 2014). Sureda et al. (2016) found HBO treatment strengthens the antioxidant capacity of plasma and contributes to the wound healing process. In our research, we discovered that HBO treatment significantly lowered the expression level of MDA and elevated the levels of SOD and GSH, which revealed that HBO treatment could suppress intestinal oxidative stress following SCI, thereby protecting intestinal barrier function.
To explore the mechanism by which HBO treatment reduced SCI-induced intestinal oxidative stress, we studied the impact of HBO on Nrf2 and its downstream genes. Nrf2 plays a critical role in alleviating oxidative stress-induced tissue injury through upregulating antioxidant enzymes. In normal circumstance, Nrf2 is bound to Kelch-like ECH-associated protein-1 (Keap1) in the cytoplasm, but upon exposure to oxidative stress, Nrf2 dissociates from Keap1and translocates into the nucleus and then induces the activation of antioxidant enzymes, for example, HO-1 and NQO1. Therefore it maintains cellular redox homeostasis (Jindam et al. 2017; Bhakkiyalakshmi et al. 2015). Accumulating evidence has revealed that HBO treatment increases Nrf2 and its targets. Meng et al. (2016) discovered that HBO treatment increased the expression levels of Nrf2 and its downstream targets HO-1 and NQO-1 after traumatic brain injury. Dhamodharan et al. (2019) demonstrated that the expression levels of Nrf2 and its target genes, including NQO-1, HO-1, and catalase (CAT), were significantly upregulated in individuals who received HBO therapy. In the rat SCI model, HBO treatment increased nitric oxide levels and activated Nrf2, which alleviated spinal neurons’ injury (Huang et al. 2016). Consistent with previous findings, in this study, the expression levels of Nrf2, HO-1, NQO-1, and GCLC in intestinal tissue were also increased following SCI, while HBO treatment further significantly increased their expression. These data suggested an important role for HBO on the activation of the Nrf2 signaling pathway in the intestine after SCI, but the detailed mechanisms still need to be further clarified.
Conclusion
To summarize, this research revealed that HBO treatment prompted the expression of intercellular junctions and reduced intestinal oxidative stress. Therefore, HBO treatment alleviated SCI-induced intestinal epithelial barrier dysfunction. Moreover, the activation of the Nrf2 signaling pathway might be involved in the improvement of intestinal epithelial barrier dysfunction by HBO treatment. These findings provide the theoretical foundation for the application of HBO treatment in intestinal injury following SCI. However, our study does have limitations. The signal cascades and regulation mechanisms involved in SCI-induced intestinal barrier dysfunction are complex. We only focused on oxidative stress and the Nrf2 signaling pathway under the exposure to HBO. Therefore, further studies are needed to investigate other related mechanisms.
References
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transaction. Exp Neurol 139:244–256. https://doi.org/10.1006/exnr.1996.0098
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta, Mol Cell Res 1865:721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Besecker EM, Deiter GM, Pironi N, Cooper TK, Holmes GM (2017) Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury. Am J Phys Regul Integr Comp Phys 312:R146–R156. https://doi.org/10.1152/ajpregu.00347
Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM (2015) The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res 91:104–114. https://doi.org/10.1016/j.phrs.2014.10.004
Chiu CJ, Scott HJ, Gurd FN (1970) Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Arch Surg 101:484–488. https://doi.org/10.1001/archsurg.1970.01340280036010
Dhamodharan U, Karan A, Sireesh D, Vaishnavi A, Somasundar A, Rajesh K, Ramkumar KM (2019) Tissue-specific role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free Radic Biol Med 138:53–62. https://doi.org/10.1016/j.freeradbiomed.2019.04.031
Ding Z, Tong WC, Lu XX, Peng HP (2014) Hyperbaric oxygen therapy in acute ischemic stroke: a review. Int Neurol 2:201–211. https://doi.org/10.1159/000362677
Ferrer MD, Sureda A, Batle JM, Tauler P, Tur JA, Pons A (2007) Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free Radic Res 41:274–281. https://doi.org/10.1080/10715760601080371
Godman CA, Joshi R, Giardina C, Perdrizet G, Hightower LE (2010) Hyperbaric oxygen treatment J induces antioxidant gene expression. Ann N Y Acad Sci 1197:178–183. https://doi.org/10.1111/j.1749-6632.2009.05393.x
Hou S, Rabchevsky AG (2014) Autonomic consequences of spinal cord injury. Comprehensive Physiology 4:1419–1453. https://doi.org/10.1002/cphy.c130045
Huang G, Diao J, Yi H, Xu L, Xu J, Xu W (2016) Signaling pathways involved in HSP32 induction by hyperbaric oxygen in rat spinal neurons. Redox Biol 10:108–118. https://doi.org/10.1016/j.redox.2016.09.011
Jin W, Wang H, Ji Y, Hu Q, Yan W, Chen G, Yin H (2008) Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine 44:135–140. https://doi.org/10.1016/j.cyto.2008.07.005
Jindam A, Yerra VG, Kumar A (2017) Nrf2: a promising trove for diabetic wound healing. Ann Transl Med 5:469. https://doi.org/10.21037/atm.2017.09.03
Liu X, Yang J, Li Z, Liang F, Wang Y, Su Q, Li C (2015) Hyperbaric oxygen treatment protects against spinal cord injury by inhibiting endoplasmic reticulum stress in rats. Spine 40:E1276–E1283. https://doi.org/10.1097/BRS.0000000000001056
Liu Y, Bao Z, Xu X, Chao H, Lin C, Li Z, Liu Y, Wang X, You Y, Liu N, Ji J (2017) Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J Neurotrauma 34:2119–2131. https://doi.org/10.1089/neu.2016.4764
Meng XE, Zhang Y, Li N, Fan DF, Yang C, Li H, Guo DZ, Pan SY (2016) Effects of hyperbaric oxygen on the Nrf2 signaling pathway in secondary injury following traumatic brain injury. Genet Mol Res 15. https://doi.org/10.4238/gmr.15016933
Novak S, Drenjancevic I, Vukovic R, Kellermayer Z, Cosic A, Tolusic Levak M, Balogh P, Culo F (2016) Anti-inflammatory effects of hyperbaric oxygenation during DSS-induced colitis in BALB/c mice include changes in gene expression of HIF-1α, proinflammatory cytokines, and antioxidative enzymes. Mediat Inflamm 2016:7141430–7141419. https://doi.org/10.1155/2016/7141430
O'Connor G, Jeffrey E, Madorma D, Marcillo A, Abreu MT, Deo SK, Dietrich WD, Daunert S (2018) Investigation of microbiota alterations and intestinal inflammation post-spinal cord injury in rat model. J Neurotrauma 35:2159–2166. https://doi.org/10.1089/neu.2017.5349
Sakoda M, Ueno S, Kihara K, Arikawa K, Dogomori H, Nuruki K, Takao S, Aikou T (2004) A potential role of hyperbaric oxygen exposure through intestinal nuclear factor-kappa B. Crit Care Med 32:1722–1729. https://doi.org/10.1097/01.ccm.0000132898.27101.6c
Sun L, Zhao L, Li P, Liu X, Liang F, Jiang Y, Kang N, Gao C, Yang J (2019) Effect of hyperbaric oxygen therapy on HMGB1/NF-κB expression and prognosis of acute spinal cord injury: a randomized clinical trial. Neurosci Lett 692:47–52. https://doi.org/10.1016/j.neulet.2018.10.059
Sureda A, Batle JM, Martorell M, Capó X, Tejada S, Tur JA, Pons A (2016) Antioxidant response of chronic wounds to hyperbaric oxygen therapy. PLoS One 11:e0163371. https://doi.org/10.1371/journal.pone.0163371
Thom SR (2011) Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 127(Suppl 1):131S–141S. https://doi.org/10.1097/PRS.0b013e3181fbe2bf
Tong LC, Wang Y, Wang ZB, Liu WY, Sun S, Li L, Su DF, Zhang LC (2016) Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol 7:253. https://doi.org/10.3389/fphar.2016.00253
Williams JM, Duckworth CA, Watson AJ, Frey MR, Miguel JC, Burkitt MD, Sutton R, Hughes KR, Hall LJ, Caamano JH, Campbell BJ, Pritchard DM (2013) A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis Model Mech 6:1388–1399. https://doi.org/10.1242/dmm.013284
Wu X, Ren J, Chen G, Wu L, Song X, Li G, Deng Y, Wang G, Gu G, Li J (2017) Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci Rep 7:4364. https://doi.org/10.1038/s41598-017-04231-5
Xu J, Huang G, Zhang K, Sun J, Xu T, Li R, Tao H, Xu W (2014) Nrf2 activation in astrocytes contributes to spinal cord ischemic tolerance induced by hyperbaric oxygen preconditioning. J Neurotrauma 31:1343–1353. https://doi.org/10.1089/neu.2013.3222
Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ, Li T, Fan J, Peng ZW, Yan WJ (2016) Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res 309:1–8. https://doi.org/10.1016/j.bbr.2016.04.045
Ying X, Tu W, Li S, Wu Q, Chen X, Zhou Y, Hu J, Yang G, Jiang S (2019) Hyperbaric oxygen therapy reduces apoptosis and dendritic/synaptic degeneration via the BDNF/TrkB signaling pathways in SCI rats. Life Sci 229:187–199. https://doi.org/10.1016/j.lfs.2019.05.029
Zhang H, Liu M, Zhang Y, Li X (2019) Trimetazidine attenuates exhaustive exercise-induced myocardial injury in rats via regulation of the Nrf2/NF-kappaB signaling pathway. Front Pharmacol 10:175. https://doi.org/10.3389/fphar.2019.00175
Zhou SH, Sun YF, Wang G (2012) Effects of hyperbaric oxygen on intestinal mucosa apoptosis caused by ischemia-reperfusion injury in rats. World J Emerg Med 3:135–140. https://doi.org/10.5847/wjem.j.1920-8642.2012.02.010
Funding
This work was supported by the Beijing Natural Science Foundation [grant numbers 7202055] and the Beijing Chaoyang Hospital 1351 Talent Training Program [grant numbers 20-1351].
Author information
Authors and Affiliations
Contributions
Conceptualization: Liu Xuehua and Yang Jing
Methodology and investigation: Liu Xuehua, Liang Fang, Song Wei, Diao Xiaoli, and Zhu Wanqiu
Data curation: Liu Xuehua and Liang Fang
Writing—original draft: Liu Xuehua
Writing—review and editing: Yang Jing
Supervision: Yang Jing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Liang, F., Song, W. et al. Effect of Nrf2 signaling pathway on the improvement of intestinal epithelial barrier dysfunction by hyperbaric oxygen treatment after spinal cord injury. Cell Stress and Chaperones 26, 433–441 (2021). https://doi.org/10.1007/s12192-020-01190-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-020-01190-1