Abstract
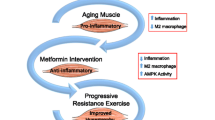
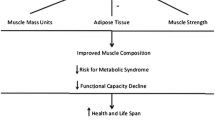
Preserving muscle mass and strength is critical for long-term health and longevity. Age-related muscle lipid accumulation has been shown to be detrimental to muscle health. In healthy older individuals, we sought to determine whether muscle lipid content, determined from computed tomography, is associated with self-reported physical function, laboratory-measured performance, and the response to progressive resistance training (PRT), and how metformin may alter these responses (N = 46 placebo, 48 metformin). Using multiple linear regression models adjusted for confounders in a large cohort, we show that intermuscular adipose tissue (IMAT) was not associated with baseline function or response to PRT, contrary to previous reports. On the other hand, thigh muscle density (TMD), as an indicator of intra- and extramyocellular lipid (IMCL and EMCL), remained strongly and independently positively associated with physical function and performance following adjustment. Baseline TMD was inversely associated with gains in strength, independent of muscle mass. Percent change in TMD was positively associated with improved chair stand and increased type II fiber frequency but was not associated with muscle hypertrophy or overall strength gain following PRT. For the first time, we show that metformin use during PRT blunted density and strength gains by inhibiting fiber type switching primarily in those with low baseline TMD. These results indicate that participants with higher muscle lipid content derive the most performance benefit from PRT. Our results further indicate that muscle density may be as influential as muscle size for strength, physical function, and performance in healthy older adults. ClinicalTrials.gov, NCT02308228, Registered on 25 November 2014







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author and, on reasonable request, a minimal de-identified dataset will be provided.
Abbreviations
- PRT:
-
progressive resistance training
- CT:
-
computed tomography
- IMAT:
-
intermuscular adipose tissue
- TMD:
-
thigh muscle density
- IMCL:
-
intramyocellular lipid
- EMCL:
-
extramyocellular lipid
- DXA:
-
dual-energy x-ray absorptiometry
- HU:
-
Hounsfield unit
- SPPB:
-
short physical performance battery
- SF-36:
-
Short Form 36
- PROMIS:
-
patient-reported outcomes measurement information system
- 1RM:
-
one repetition max
- CSA:
-
cross-sectional area
References
Addison OR (2013) Fit or fat: the relationship of inflammation, intramuscular fat and muscle, and mobility function in older adults. ProQuest Information & Learning.
Addison O, Wende AR, McClain DA, Marcus RL, Drummond MJ, Lastayo PC, Dibble LE (2014) Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity [electronic resource] journal of nutrition, health & aging 18:532-538 https://doi.org/10.1007/s12603-014-0019-1.
Bamman MM, Petrella JK, Jeong-su K, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. Journal of Applied Physiology. 2007;102:2232–9.
Barzilay JI, Cotsonis GA, Walston J, Schwartz AV, Satterfield S, Miljkovic I, Harris TB (2009) Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged ≥ 70 years Diabetes Care 32:736-738 doi:https://doi.org/10.2337/dc08-1781.
Boule NG, Kenny GP, Larose J, Khandwala F, Kuzik N, Sigal RJ. Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Diabetologia. 2013;56:2378–82. https://doi.org/10.1007/s00125-013-3026-6.
Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, et al. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiologica. 2016;216:15–41. https://doi.org/10.1111/apha.12532.
Camera DM. Anabolic heterogeneity following resistance training: a role for circadian rhythm? Front Physiol. 2018;9:569. https://doi.org/10.3389/fphys.2018.00569.
Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–9. https://doi.org/10.1111/j.1532-5415.2009.02366.x.
Cella D et al. (2019) PROMIS ® adult health profiles: efficient short-form measures of seven health domains value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research 22:537-544 https://doi.org/10.1016/j.jval.2019.02.004.
Chmelo EA, Crotts CI, Newman JC, Brinkley TE, Lyles MF, Leng X, et al. Heterogeneity of physical function responses to exercise training in older adults. Journal of the American Geriatrics Society. 2015;63:462–9. https://doi.org/10.1111/jgs.13322.
Choi SJ, et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2016;71:557–64.
Churchward-Venne TA, Breen L, Phillips SM. Alterations in human muscle protein metabolism with aging: protein and exercise as countermeasures to offset sarcopenia. Biofactors (Oxford, England). 2014;40:199–205. https://doi.org/10.1002/biof.1138.
Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LCPGM, et al. There are no nonresponders to resistance-type exercise training in older men and women. Journal Of The American Medical Directors Association. 2015;16:400–11. https://doi.org/10.1016/j.jamda.2015.01.071.
Cleary LC, Crofford LJ, Long D, Charnigo R, Clasey J, Beaman F, et al. Does computed tomography-based muscle density predict muscle function and health-related quality of life in patients with idiopathic inflammatory myopathies? Arthritis Care & Research. 2015;67:1031–40. https://doi.org/10.1002/acr.22557.
Delmonico MJ, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. American Journal of Clinical Nutrition AJN. 2009;90:1579–85.
Frank-Wilson AW, et al. Associations of quadriceps torque properties with muscle size, attenuation, and intramuscular adipose tissue in older adults. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2018;73:931–8.
Garatachea N, Lucía A. Genes and the ageing muscle: a review on genetic association studies. Age. 2013;35:207–33. https://doi.org/10.1007/s11357-011-9327-0.
Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. Journal of Applied Physiology (Bethesda, Md: 1985). 2000;89:104–10.
Guralnik JM. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61.
Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Physical Therapy. 2008;88:1336–44. https://doi.org/10.2522/ptj.20080079.
Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2012;67:1140–52.
Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Medicine. 2004;34:329–48.
Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN Journal of Parenteral & Enteral Nutrition. 2008;32:656–9.
Khoja SS, Moore CG, Goodpaster BH, Delitto A, Piva SR. Skeletal muscle fat and its association with physical function in rheumatoid arthritis. Arthritis Care & Research. 2018;70:333–42. https://doi.org/10.1002/acr.23278.
Konopka AR et al. (2018) Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults Aging Cell:e12880 https://doi.org/10.1111/acel.12880.
Kulkarni AS et al. (2020) Metformin alters skeletal muscle transcriptome adaptations to resistance training in older adults aging accepted.
Larson-Meyer DE, Ravussin E, Newcomer BR, Kelley DE, Smith SR, Heilbronn LK. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity research. 2006;14:73–87.
Law TD, Clark LA, Clark BC. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annual review of gerontology & geriatrics. 2016;36:205–28.
Long DE, et al. Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials. 2017;18:1–14. https://doi.org/10.1186/s13063-017-1932-5.
Long DE, Villasante Tezanos AG, Wise JN, Kern PA, Bamman MM, Peterson CA, et al. A guide for using NIH Image J for single slice cross-sectional area and composition analysis of the thigh from computed tomography. PLoS ONE. 2019;14:1–11. https://doi.org/10.1371/journal.pone.0211629.
Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131–6. https://doi.org/10.2337/dc11-0925.
Marcus RL, Addison O, Dibble LE, Bo Foreman K, Morrell G, LaStayo P (2012) Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals Journal of Aging Research:1-6 https://doi.org/10.1155/2012/629637.
McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993;31:247–63.
Miljkovic N, Lim J-Y, Miljkovic I, Frontera WR. Aging of skeletal muscle fibers. Annals Of Rehabilitation Medicine. 2015;39:155–62. https://doi.org/10.5535/arm.2015.39.2.155.
Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Frontiers In Physiology. 2012;3:260. https://doi.org/10.3389/fphys.2012.00260.
Moretti I et al. (2016) MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity Nature Communications 7:12397-12397 https://doi.org/10.1038/ncomms12397.
Petrella JK, Jeong-su K, Tuggle SC, Hall SR, Bamman MM (2005) Age differences in knee extension power, contractile velocity, and fatigability Journal of Applied Physiology 98:211-220.
Petrella JK, Kim J-S, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. American Journal Of Physiology Endocrinology And Metabolism. 2006;291:E937–46.
Rahemi H, Nigam N, Wakeling JM (2015) The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese Journal Of The Royal Society, Interface 12:20150365–20150365 https://doi.org/10.1098/rsif.2015.0365.
Rastelli F et al. (2015) Effects of muscle composition and architecture on specific strength in obese older women Experimental Physiology 100:1159-1167 https://doi.org/10.1113/EP085273.
Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exercise & Sport Sciences Reviews. 2012;40:4–12.
Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA (2016) Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity American journal of physiology regulatory, integrative and comparative physiology 310:R561-R569 https://doi.org/10.1152/ajpregu.00198.2015.
Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metabolism. 2017;25:581–92. https://doi.org/10.1016/j.cmet.2017.02.009.
Saisho Y (2015) Metformin and inflammation: its potential beyond glucose-lowering effect endocrine, metabolic & immune disorders drug targets 15:196-205.
Sayiner ZA, Öztürk ZA (2019) Relationship between sarcopenia and type 2 diabetes mellitus in elderly patients Yaşlı Hastalarda Sarkopeni ve Tip 2 Diabetes Mellitus Arasındaki İlişki 23:47-52 https://doi.org/10.25179/tjem.2018-62624.
Schering L et al. (2015) Identification of novel putative adipomyokines by a cross-species annotation of secretomes and expression profiles Archives of Physiology & Biochemistry 121:194-205 https://doi.org/10.3109/13813455.2015.1092044.
Scott D et al. (2015) Associations of calf inter- and intra-muscular adipose tissue with cardiometabolic health and physical function in community-dwelling older adults journal of Musculoskeletal & Neuronal Interactions 15:350-357.
Srikuea R et al. (2013) Association of fibromyalgia with altered skeletal muscle characteristics which may contribute to postexertional fatigue in postmenopausal women Arthritis And Rheumatism 65:519-528 https://doi.org/10.1002/art.37763.
Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. American Journal Of Physiology Endocrinology And Metabolism. 2016;310:E652–61. https://doi.org/10.1152/ajpendo.00486.2015.
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The Journals Of Gerontology Series A, Biological Sciences And Medical Sciences. 2014;69:547–58. https://doi.org/10.1093/gerona/glu010.
Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55:217–23. https://doi.org/10.1159/000182084.
Trombetti A, Reid K, Pasha E, Phillips E, Fielding R, Hars M, Herrmann F (2016) Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life Osteoporosis International 27:463-471 https://doi.org/10.1007/s00198-015-3236-5.
Visser M, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. Journals of Gerontology Series A: Biological Sciences & Medical Sciences. 2005;60:324–33.
Walton RG et al. (2019) Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial Aging Cell:e13039 https://doi.org/10.1111/acel.13039.
Yuan W et al. (2018) MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry Journal of Applied Physiology 124:40-51.
Acknowledgments
The authors would like to thank each of our valuable research participants for their time, effort, and dedication. We would like to thank Tara Bennett PA-C for performing muscle biopsies and Janna Jackson PhD and Cory Dungan PhD for performing immunohistochemistry. We would also like to thank Ameya Kulkarni, PhD and Nir Barzilai, MD, PhD of the Albert Einstein School of Medicine Nathan Shock Center for assistance with RNA sequencing.
Funding
The study was funded by the National Institutes of Health - National Institute on Aging grant AG046920 and supported by the NIH Clinical and Translational Science Awards (CTSA) (UL1TR001998) at the University of Kentucky and the NIH CTSA (UL1TR000165) at the University of Alabama at Birmingham. This study was also supported by an award from the Glenn Foundation for Medical Research.
Author information
Authors and Affiliations
Contributions
DEL, CAP, and RGW wrote the manuscript; DEL, PAK, MMB, CAP, and RGW contributed to the design of the study; DEL, SCT, STW, MMB, PAK, and CAP implemented the clinical protocol; DEL, BCP, and RGW performed analyses; AGV provided statistical analysis of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the University of Kentucky institutional review board (IRB 14-0330) and the University of Alabama at Birmingham institutional review board (IRB F140722001) prior to any participants enrolling. Data and safety monitoring was provided by the UK CCTS DSMB on a quarterly basis.
Consent to participate
All participation was on a voluntary basis, and each participant was required to sign an approved IRB consent form and HIPAA authorization prior to any procedures taking place.
Consent for publication
Not applicable.
Disclaimer
The NIH had no role in the design of the study, collection, analysis, or interpretation of data, or in writing the manuscript.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The first author, DEL, is a research coordinator in the University of Kentucky College of Health Sciences, and an exercise physiologist in the UK CCTS Functional Assessment and Body Composition Core Lab.
Supplementary information
Figure s1
Mid-thigh intermuscular adipose tissue (IMAT) is inversely correlated with thigh muscle density (TMD) at baseline HU, Hounsfield Unit (PDF 97 kb)
Figure s2
Metformin blunts loss in IMAT following resistance training (PDF 93 kb)
Figure s3
Metformin preferentially inhibits performance gains in those with high amounts of baseline thigh intermuscular adipose tissue (IMAT) for (b, c) dynamic and isometric strength but not a power. *p < 0.05 (PDF 119 kb)
Table s1
(DOCX 16 kb)
Table s2
(DOCX 18 kb)
Table s3
Baseline gene expression in high vs. low TMD. See electronic supplementary excel file- intended for publication as an online data supplement (XLSX 28 kb)
Table s4
Correlations between gene fold change and % change in TMD in the placebo group following PRT (XLSX 24.8 kb)
About this article
Cite this article
Long, D.E., Peck, B.D., Tuggle, S.C. et al. Associations of muscle lipid content with physical function and resistance training outcomes in older adults: altered responses with metformin. GeroScience 43, 629–644 (2021). https://doi.org/10.1007/s11357-020-00315-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00315-9