Abstract
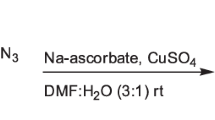
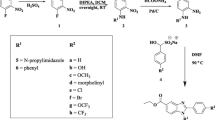
Searching for new active molecules against M. Bovis BCG and Mycobacterium tuberculosis (MTB) H37Ra, a focused of 1,2,3-triazoles-incorporated 2,4 thiazolidinedione conjugates have been efficiently prepared via a click chemistry approach cyclocondensation of 4-amino-N-(5-methylisoxazol-3-yl)benzenesulfonamide (4), aryl aldehyde (5a–l), and mercapto acetic acid (6) with good to promising yields. The newly synthesized compounds were tested against drug-sensitive MTB and BCG. In particular, compounds 8g, 8h, 8j and 8l are highly potent against both the strains with IC90 values in the range of 1.20–2.70 and 1.24–2.65 µg/mL, respectively. Based on the results from the antitubercular activity, SAR for the synthesized series has been developed. Most of the active compounds were non-cytotoxic against MCF-7, HCT 116 and A549 cell lines. Most active compounds were having a higher selectively index, which suggested that these compounds were highly potent.





Similar content being viewed by others
References
C. Dye, B.G. Williams, Science 328, 856 (2010)
WHO Global Tuberculosis Report 2014; World Health Organization: Geneva, 2014; http://www.who.int/tb/publications/ global report/en/
S. Loewenberg, Lancet 379, 205 (2012)
A.I. Zumla, S.H. Gillespie, M. Hoelscher, P.P. Philips, S.T. Cole, I. Abubakar, T.D. McHugh, M. Schito, M. Maeurer, A.J. Nunn, Lancet Infect. Dis. 4, 327 (2014)
CDC, 2011, MMWR Morb Mortal Wkly Rep. 61, 883 (2012)
R.J. Rees, Br. Med. Bull. 25, 183 (1969)
Z.F. Udwadia, R.A. Amale, K.K. Ajbani, C. Rodrigues, Clin. Infect. Dis. 54, 579 (2012)
M.S. Butler, M.A. Blaskovich, M.A. Cooper, J Antibiot (Tokyo) 66, 571 (2013)
A.H. Diacon, P.R. Donald, A. Pym, M. Grobusch, R.F. Patientia, R. Mahanyele, N. Bantubani, R. Narasimooloo, T. De Marez, R. van Heeswijk, Antimicrob. Agents Chemother. 6, 3271 (2012)
A. Massarotti, S. Aprile, V. Mercalli, E.D. Grosso, G. Grosa, G. Sorba, G.C. Tron, Chem. Med. Chem. 9, 2497 (2014)
K.D. Hanni, D.A. Leigh, Chem. Soc. Rev. 39, 1240 (2010)
K. Kempe, A. Krieg, C.R. Becer, U.S. Schubert, Chem. Soc. Rev. 41, 176 (2012)
A.D. Moorhouse, J.E. Moses, Chem. Med. Chem. 3, 715 (2008)
R.D. Simone, M.G. Chini, I. Bruno, R. Riccio, D. Mueller, O. Werz, G. Bifulco, J. Med. Chem. 54, 1565 (2011)
B. Zhang, Eur. J. Med. Chem. 168, 357 (2019)
N. Fu, S. Wang, Y. Zhang, C. Zhang, D. Yang, L. Weng, B. Zhao, L. Wang, Eur. J. Med. Chem. 136, 596 (2017)
S.G. Agalave, S.R. Maujan, V.S. Pore, Chem. Asian J. 6, 2696 (2011)
S. Hakimian, A. Cheng-Hakimian, G.D. Anderson, J.W. Miller, Expert Opin. Pharmacother. 8, 1931 (2007)
S. Zhang, Z. Xu, C. Gao, Q.C. Ren, L. Chang, Z.S. Lv, L.S. Feng, Eur. J. Med. Chem. 138, 501 (2017)
N. Boechat, V.F. Ferreira, S.B. Ferreira, M.L.G. Ferreira, F.C. da Silva, M.M. Bastos, M.S. Costa, M.C.S. Lourenço, A.C. Pinto, A.U. Krettli, A.C. Aguiar, B.M. Teixeira, N.V. da Silva, P.R.C. Martins, F.A.F.M. Bezerra, A.L.S. Camilo, G.P. da Silva, C.C.P. Costa, J. Med. Chem. 54, 5988 (2011)
C. Menendez, S. Gau, C. Lherbet, F. Rodriguez, C. Inard, M.R. Pasca, M. Baltas, Eur. J. Med. Chem. 46, 5524 (2011)
S. R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, T. Anjana Devi, S. V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911 (2012)
Y. Tian, Z. Liu, J. Liu, B. Huang, D. Kang, H. Zhang, E.D. Clercq, D.D. Mans, C. Pannecouque, K.H. Lee, C.H. Chen, P. Zhan, X. Liu, Eur. J. Med. Chem. 151, 339 (2018)
M. Allam, A.K.D. Bhavani, A. Mudiraj, N. Ranjan, M. Thippana, P.P. Babu, Eur. J. Med. Chem. 156, 43 (2018)
Y. Chinthala, A. Kumar, A.D. Sarfaraz, S.P. Singh, N.K. Arigari, N. Gupta, S.K.V.N. Satya, J.K. Kumar, F. Khan, A.K. Tiwari, G. Paramjit, Eur. J. Med. Chem. 70, 308 (2013)
S. Rekha, U. Shantharam, V. Chandy, Int. Res. J. Pharm. 2, 81 (2011)
J. Sindhu, H. Singh, J.M. Khurana, C. Sharma, K.R. Aneja, Chin. Chem. Lett. 26, 50 (2015)
S. Ponnuchamy, S. Kanchithalaivan, R.R. Kumar, M.A. Ali, T.S. Choon, Bioorg. Med. Chem. Lett. 24, 1089 (2014)
D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, C.W. Day, D.F. Smee, P. Grellier, R. Lesyk, Eur. J. Med. Chem. 66, 228 (2013)
B.R. Bhattarai, B. Kafle, J.S. Hwang, S.W. Ham, K.H. Lee, H. Park, I.O. Han, H. Cho, Bioorg. Med. Chem. Lett. 20, 6758 (2010)
X. Li, R.K. Russell, J. Spink, S. Ballentine, C. Teleha, S. Branum, K. Wells, D. Beauchamp, R. Patch, H. Huang, M. Player, W. Murray, Org. Process Res. Dev. 18, 321 (2014)
A.M. Ali, G.E. Saber, N.M. Mahfouz, M.A. El-Gendy, A.A. Radwan, M.A. Hamid, Arch. Pharm. Res. 30, 1186 (2007)
L.A. Dakin, M.H. Block, H. Chen, E. Code, J.E. Dowling, X. Feng, A.D. Ferguson, I. Green, A.W. Hird, T. Howard, E.K. Keeton, M.L. Lamb, P.D. Lyne, H. Pollard, J. Read, A.J. Wu, T. Zhang, X. Zheng, Bioorg. Med. Chem. Lett. 22, 4599 (2012)
D.O. Bozdag, E.J. Verspohl, E.N. Das, R.M. Kaup, K. Bauer, M. Sarıkaya, B. Evranos, R. Ertan, Bioorg. Med. Chem. 16, 6747 (2008)
A.K.M. Iqbal, A.Y. Khan, M.B. Kalashetti, N.S. Belavagi, Y.D. Gong, I.A.M. Khazi, Eur. J. Med. Chem. 53, 308 (2012)
N. Zidar, T. Tomasic, R. Sink, A. Kovac, D. Patin, D. Blanot, C. Contreras- Martel, A. Dessen, M. M. Premru, A. Zega, S. Gobec, L. P. Masic, D. Kikelj, Eur. J. Med. Chem. 46, 5512 (2011)
F. W. Barros, T. G. Silva, M. G. da Rocha Pitta, D. P.Bezerra, L. V. Costa-Lotufo, M. O. de Moraes, C. Pessoa, M. A. de Moura, F. C. de Abreu, C. de Lima Mdo, S. L. Galdino, R. Pitta Ida, M. O. Goulart, Bioorg. Med. Chem., 20, 3533 (2012)
Z. Xia, C. Knaak, J. Ma, Z.M. Beharry, C. McInnes, W. Wang, A.S. Kraft, C.D. Smith, J. Med. Chem. 52, 74 (2009)
F.H.A. Leite, P.B.G.S. Santiago, T.Q. Froes, J.S. Filho, S.G. Silva, R.M. Ximenes, A.R. Faria, D.J. Brondani, J.F.C. Albuquerque, M.S. Castilho, Eur. J. Med. Chem. 123, 639 (2016)
K. Liu, W. Rao, H. Li, Q. Parikh, T.L. Guo, S. Grant, G.E. Kellogg, S. Zhang, Eur. J. Med. Chem. 47, 125 (2012)
A. Maleki, A. Sarvary, RSC Adv. 5, 60938 (2015)
A. Maleki, V. Eskandarpour, Jour. of Iran. Chem. Soc. 16, 1459 (2019)
A.T. Kal-Koshvandi, M.R. Ahghari, A. Maleki, New J. Chem. 44, 12619 (2020)
A. Maleki, M. Panahzadeh, R. Eivazzadeh-keihan, Green Chem. Lett and Rev. 12, 395 (2019)
A.A. Ali, M. Konwar, M. Chetia, D. Sarma, Tetrahedron Lett. 57, 5661 (2016)
M. Tavassoli, A.L. Isfahani, M. Moghadam, S. Tangestaninejad, V. Mirkhani, I.M. Baltork, ACS Sustainable Chem. Eng. 4, 1454 (2016)
M. Dabiri, S.K. Movahed, D.I. MaGee, Res. Chem. Intermed. 41, 3335 (2015)
H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Eur. J. Med. Chem. 77, 145 (2014)
A.Z. Ahmady, F. Heidarizadeh, M. Keshavarz, Synth. Commun. 43, 2100 (2013)
A. Marra, A. Vecchi, C. Chiappe, B. Melai, A. Dondoni, J. Org. Chem. 73, 2458 (2008)
Y.B. Zhao, Z.Y. Yan, Y.M. Liang, Tetrahedron Lett. 47, 1545 (2006)
G. Wang, Z. Peng, J. Wang, X. Li, J. Li, Eur. J. Med. Chem. 125, 423 (2017)
C. Ferroni, A. Pepe, Y. Sang Kim, S. Lee, A. Guerrini, M. D. Parenti, A. Tesei, A. Zamagni, M. Cortesi, N. Zaffaroni, M. De Cesare, G. L. Beretta, J. B. Trepel, S. V. Malhotra, G. Varchi, J. Med. Chem. 60, 3082 (2017)
K.C. Tiew, D. Dou, T. Teramoto, Bioorg. Med. Chem. 20, 1213 (2012)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2596 (2002)
J. Sultana, N.D. Khupse, S. Chakrabarti, P. Chattopadhyay, D. Sarma, Tetrahedron Lett. 60, 1117 (2019)
Z. Zhang, Q. Zhou, F. Ye, Y. Xia, G. Wu, M.L. Hossain, Y. Zhang, J. Wang, Adv. Synth. Catal. 357, 2277 (2015)
D. Gangaprasad, J. Paul Raj, T. Kiranmye, K. Karthikeyan, J. Elangovan, European J. Org. Chem. 2016, 5642 (2016)
Y.Y. Xie, Y.C. Wang, Y. He, D.C. Hu, H.S. Wang, Y.M. Pan, Green Chem. 19, 656 (2017)
I. Proietti Silvestri, F. Andemarian, G. N. Khairallah, S. Wan Yap, T. Quach, S. Tsegay, C. M. Williams, R. A. J. O’Hair, P. S. Donnelly, S. J. Williams, Org. Biomol. Chem. 9, 6082 (2011)
W.S. Brotherton, R.J. Clark, L. Zhu, J. Org. Chem. 77, 6443 (2012)
K. Yamamoto, T. Bruun, J.Y. Kim, L. Zhang, M. Lautens, Org. Lett. 18, 2644 (2016)
I. E. Pardini, M. Gagliardi, M. C. Colone, M. Stringaro, A. R. Teloni, R. Brunori, L. Nisini, R. Fattorini, L. Giannoni, F. Microbes Infect. 14, 959 (2012) and references cited therin
A. A. Van de Loosdrecht, R. H. Beelen, G. J. Ossenkoppele, M. G. Broekhoven, M. M, Langenhuijsen, J. Immunol. Methods, 17, 311 (1994)
R.C. Hartkoorn, B. Chandler, A. Owen, S.A. Ward, S.B. Squire, D.J. Back, S.H. Khoo, Tuberculosis 87, 248 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Rights and permissions
About this article
Cite this article
Kulkarni, P.S., Karale, S.N., Khandebharad, A.U. et al. Synthesis of novel 1,2,3-triazoles bearing 2,4 thiazolidinediones conjugates and their biological evaluation. J IRAN CHEM SOC 18, 2035–2046 (2021). https://doi.org/10.1007/s13738-021-02160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02160-9