Abstract
Antisocial personality disorder (ASPD) imposes a high societal burden given the repetitive reactive aggression that affected individuals perpetrate. Since the brain endocannabinoid system (ECS) has been implicated in ASPD and aggressive behavior, we utilized [11C]CURB positron emission tomography to investigate fatty acid amide hydrolase (FAAH), an enzyme of the ECS that degrades anandamide, in 16 individuals with ASPD and 16 control participants. We hypothesized that FAAH density would be lower in the amygdala for several reasons. First, decreased FAAH expression is associated with increased cannabinoid receptor 1 stimulation, which may be responsible for amygdala hyper-reactivity in reactive aggression. Second, the amygdala is the seat of the neural circuit mediating reactive aggression. Third, other PET studies of externalizing populations show reduced brain FAAH density. Conversely, we hypothesized that FAAH expression would be greater in the orbitofrontal cortex. Consistent with our hypothesis, we found that amygdala FAAH density was lower in the amygdala of ASPD (p = 0.013). Cerebellar and striatal FAAH expression were inversely related with impulsivity (cerebellum: r = −0.60, p = 0.017; dorsal caudate: r = −0.58, p = 0.023; dorsal putamen: r = −0.55, p = 0.034), while cerebellar FAAH density was also negatively associated with assaultive aggression (r = −0.54, p = 0.035). ASPD presents high levels of disruptive behavior with few, if any, efficacious treatment options. Novel therapeutics that increase FAAH brain levels in a region-specific manner could hold promise for attenuating certain symptom clusters of ASPD, although our results require replication.
Similar content being viewed by others
Introduction
Antisocial personality disorder (ASPD) is a neurodevelopmental condition characterized by reckless, aggressive, and impulsive behavior1. Half of all individuals with ASPD possess a record of criminal offending, and 85% have acted violently toward others2,3. ASPD is also associated with the highest risk of victimizing children and strangers among all psychiatric disorders4. Many mental disorders are often comorbid with ASPD, such as schizophrenia (SCZ) or psychopathy, which portends a poorer overall prognosis5. Despite advances in our clinical knowledge of ASPD, comparatively little is known about its neurobiology.
A key feature of ASPD is high endorsement of reactive or impulsive aggression, which is aggression enacted in response to provocative or threatening stimuli. The neural circuit that mediates reactive aggression has been localized to the amygdala, hypothalamus, and periaqueductal gray6. A combination of increased, hyperactive limbic (e.g., amygdala) structures in concert with decreased orbitofrontal cortex (OFC) activity is a highly-regarded hypothesis of the mechanism underlying reactive aggression7. Studies of reactively aggressive populations, including individuals with intermittent explosive disorder8, impulsively aggressive spouse abusers9, and reactively aggressive violent offenders10 all report increased amygdala responsiveness to threatening stimuli. Since the OFC and amygdala share numerous reciprocal connections that influence impulsivity11, there is a need to prioritize research of these regions in neurobiological studies of ASPD.
Our understanding of the molecular underpinnings of ASPD is particularly limited. To our knowledge, there are no postmortem studies of individuals with the clinical diagnosis. Moreover, extant brain positron emission tomography (PET) studies have primarily focused on monoaminergic neurotransmission in relation to the diagnosis or characteristics of the disorder12. Fortunately, new positron emission tomography (PET) radiotracers have been developed that can radiolabel core constituents of the endocannabinoid system (ECS) in vivo13. This advance is noteworthy, since the ECS has been implicated in the pathophysiology of ASPD and aggressive behavior14, although data linking the ECS to impulsive aggression have been mixed and largely limited to animal models15.
Composed of cannabinoid receptors, endocannabinoid (EC) ligands, and enzymes that regulate EC function, a chief function of the ECS is to modulate cognitive processes and social behaviors16. One molecule of interest is the enzyme fatty acid amide hydrolase (FAAH). Located post-synaptic to the cannabinoid 1 receptor (CB1), FAAH metabolizes the endocannabinoid N-arachidonoylethanolamine (anandamide; AEA). AEA is an EC that principally binds to CB117. In a unique signaling pathway, AEA is synthesized on-demand in post-synaptic neurons before engaging in retrograde feedback onto pre-synaptic CB118. Molecules that regulate AEA are thus able to indirectly control CB1 signaling and presumably modulate socioemotional behaviors, including aggression19.
Boileau and colleagues were the first to discover that a single nucleotide polymorphism of the FAAH gene (rs324420), which involves transversion of a cytosine residue to the nucleoside adenine (C385A), affects the kinetics of [11C]CURB, a PET radiotracer that can quantify FAAH density. Both C/A and A/A genotypes have lower production of in vivo brain FAAH expression relative to those with the C/C genotype20. Interestingly, in a sample of Spanish males with alcohol dependence, an association between ASPD and greater frequency of the C385A genotype emerged, suggesting lower brain levels of FAAH in ASPD14. Lower brain FAAH expression is consistent with the notion of higher brain levels of AEA and subsequently greater CB1 activation. Here, we suggest that hyperactivity of the amygdala observed in reactively aggressive populations may result from increased CB1 neurotransmission as a result of lower FAAH density. On the contrary, increased OFC activity may stem in part from higher FAAH levels and decreased CB1 activation, consistent with recent findings of another study that found elevated PFC FAAH density in an aggressive population21. Application of [11C]CURB PET, a radioligand with high specificity for the FAAH enzyme13, could extend functional magnetic resonance imaging findings in reactively aggressive ASPD to contribute plausible neurochemical mechanisms explaining the disorder and pathological core traits.
Our primary objective was to assay FAAH expression in the amygdala and OFC of reactively aggressive violent offenders with ASPD, although we also conducted a whole-brain analysis. We selected for primary analysis the amygdala and OFC, given their putative involvement in the commission of reactive aggression and the fact that they have shown structural and functional abnormalities in ASPD and antisocial populations22. We included ASPD individuals with and without comorbid SCZ to increase the generalizability of results. For example, although ASPD is very common in forensic psychiatric patients who have primary psychotic disorders, the comorbid conditions have rarely, if ever, been investigated in PET investigations. We hypothesized that amygdala FAAH expression would be lower in ASPD with reactive aggression and higher in the OFC of reactively aggressive ASPD. We further hypothesized that measures of impulsivity and aggression would correlate with FAAH density in the brain regions of interest.
Patients and methods
All participants provided written informed consent after all study components were fully explained to them. The Research Ethics Board of the Centre for Addiction and Mental Health (CAMH) in Toronto, Ontario, Canada, approved all procedures of this investigation.
Participants
Sixteen patients with ASPD (ASPD-SCZ = 11; ASPD + SCZ = 5) and a history of violent offending and 16 control participants (healthy controls = 11; SCZ = 5) with no history of violent offending completed the investigation. The sample size chosen to detect a pre-specified effect was based on similar sample sizes of recent PET experiments23. Inclusion and exclusion criteria were pre-determined. Experimental participants and controls were sex-matched (all male). All diagnoses were verified according to results from the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II)24 and Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)25 (for the ASPD and healthy controls) by trained raters. In addition, ASPD diagnoses were reviewed and confirmed by a forensic psychiatrist experienced in the assessment and treatment of personality disorders (NJK).
ASPD-SCZ
ASPD-SCZ participants were recruited from the local Toronto community and correctional centers. Exclusion criteria for the ASPD-SCZ participants included a current major depressive episode (MDE); history of mania, hypomania, or psychotic illness; and diagnosis of substance abuse or dependence in the past 12 months as confirmed by the SCID-I. The use of psychotropic medications or herbs in the past three months was also exclusionary. One ASPD-SCZ participant was a smoker. For all study groups (ASPD-SCZ; ASPD + SCZ; SCZ; and healthy controls), neurological illness; head trauma; positive drug screen for drugs of abuse, including cannabis, on scan and assessment days; and contraindications to safe magnetic resonance imaging (MRI) scanning also precluded participation.
ASPD + SCZ
ASPD + SCZ participants were recruited from forensic psychiatry inpatient and outpatient services at CAMH. Exclusion criteria for the ASPD + SCZ participants included an MDE and/or substance use disorder in the past 12 months. Three ASPD + SCZ participants were current smokers. For all ASPD participants (ASPD-SCZ and ASPD + SCZ), the urine drug screen utilized was Rapid ResponseTM, Drugs of Abuse Test Panel (BTNX Inc., Markham, Ontario, Canada). It tests for the presence of opiates, phencyclidine, barbiturates, benzodiazepines, tricyclic antidepressants, amphetamines, tetrahydrocannabinol, and methadone.
SCZ
SCZ participants were recruited from outpatient programs at a tertiary care psychiatric center. They were selected from a larger group of SCZ patients, because they had not used illicit substances, including cannabis. Exclusion criteria for the SCZ participants included a positive drug screen at assessment and scanning days and a diagnosis of substance abuse or dependence. None of the SCZ participants were smokers. The drug screen used for SCZ participants was a urine immunoassay administered at the CAMH laboratory that tested for the presence of ethanol, methadone, benzodiazepines, tetrahydrocannabinol, opiates, and cocaine metabolites.
Healthy controls
Healthy controls had no history of psychiatric illness according to SCID-I and SCID-II reports. They were recruited from the community. All were non-smokers. The drug screen used for healthy participants was manufactured by BTNX (BTNX Inc., Markham, Ontario, Canada). It tests for the presence of cocaine, amphetamines, opiates, oxycodone, methadone, 3,4-methylenedioxymethamphetamine, benzodiazepines, ketamine, tetrahydrocannabinol, and tricyclic antidepressants.
All study participants were asked to refrain from using alcohol the night before and the day of PET scanning.
Image acquisition and analysis
Each participant completed one [11C]CURB PET scan at the CAMH Brain Health Imaging Centre. Radiosynthesis of [11C]CURB has been previously described26. Participants underwent PET utilizing a three-dimensional HRRT brain tomograph (CPS/Siemens, Knoxville, TN, USA). For the duration of the scan, participants lay on their backs and wore a thermoplastic mask to reduce movement. A transmission scan was conducted followed by injection of 370 ± 40 MBq (10 ± 1 mCi) of [11C]CURB27. An advantage of [11C]CURB is its high specific binding13. Brain radioactivity was computed during sequential frames of increasing duration, and the scan time was 60 min. PET images were next re-constructed using a filtered back-projection algorithm with a HANN filter at Nyquist cutoff frequency28. Arterial samples were sampled continuously for the first 22.5 min with an automatic blood sampling system (Model PBS-101, Veenstra Instruments, Joure, The Netherlands) after [11C]CURB injection. Radioactivity in whole blood and plasma (1500 relative centrifugal force, 5 min) was counted utilizing a Packard Cobra II or Wizard 2480 γ-counter (Packard Instrument Co., Meridian, CT, USA) cross-calibrated with the PET system. The concentration of parent radioligand and its metabolites was measured in each manual sample (except for the one at 15 min) as previously validated26. Blood-to-plasma radioactivity ratios were fitted utilizing a biexponential function and parent plasma fraction using a Hill function. A dispersion- and metabolite-corrected arterial plasma input function was generated as previously described26.
Each participant received a standard proton-density weighted brain MRI scan (TE = 17, TR = 6000, FOV = 22 cm, matrix = 256 × 256, slice thickness = 2 mm; number of excitations = 2) acquired on a Discovery MR750 3 T MRI scanner (General Electric, Milwaukee, WI, USA) for region of interest (ROI) delineation. ROIs were generated automatically using in-house software (ROMI) as previously reported29. Time-activity curves were acquired over 60 min. in each of the ROIs and analyzed by a two-tissue compartment model with irreversible binding to the second component26. FAAH binding was quantified using the composite parameter λk3 (λ = K1/k2).
FAAH polymorphism genotyping
For all study participants, the FAAH rs324420 variant was genotyped according to the manufacturer’s directions for a TaqMan SNP Genotyping assay (ID C_1897306_10; Life Technologies, Burlington, Canada) on a ViiA7 instrument (Life Technologies, Burlington, Canada) using 20 ng total genomic DNA template, Perfecta FastMix II (Quantabio, Beverly, MA, USA), in a total reaction volume of 10 μL as previously performed20.
Instruments
ASPD subjects completed the Urgency, Premeditation, Perseverance, Sensation Seeking, Positive Urgency Impulsive Behavior Scale (UPPS-P)30 to quantify facets of impulsivity; the Buss-Durkee Hostility Index (BDHI)31 to assess levels of hostility and aggression; and the Reactive-Proactive Aggression Questionnaire32 to measure levels of reactive aggression; the Psychopathy Checklist: Screening Version (PCL:SV)33 and Triarchic Psychopathy Measure (TriPM)34 to gauge the presence of psychopathic traits; and the State-Trait Anxiety Inventory (STAI)35 to measure anxiety symptoms.
Statistical analysis
Sample demographic and clinical information were compared between groups using chi-square tests, independent samples t-tests, and independent samples Mann–Whitney U tests. All tests of significance were two-sided. The Shapiro–Wilks test was used to assess the normality of data for all comparisons of continuous data. Non-parametric tests were utilized where data violated the assumptions of normality. To compare [11C]CURB λk3 between groups (ASPD + SCZ and ASPD-SCZ versus SCZ and healthy controls), we employed multivariate analysis of covariance (MANCOVA) with ASPD diagnosis as a between-subjects factor and genotype as a covariate. The main model that was used to test our primary hypothesis included two ROIs: OFC and amygdala. Main effects were analyzed by univariate ANOVA with a p-value < 0.05 indicating significance. Our secondary model tested the whole brain, including anterior cingulate cortex (ACC), temporal cortex, insula, hippocampus, thalamus, ventral striatum, dorsal putamen, dorsal caudate, and cerebellum (11 ROIs). Bonferroni corrections were applied (0.05/9 secondary regions = 0.0056) to correct for multiple comparisons.
Partial correlations between UPPS-P and BDHI scores with FAAH binding in our ROIs controlling for FAAH genotype were planned a priori and were not adjusted for multiple comparisons. For partial correlations examining psychopathic traits and anxiety symptoms, Bonferroni correction was applied. Data in Table 1 are presented as means and standard deviations.
Results
Subject characteristics
Participants’ clinical and demographic information is reported in Table 1.
Information on comorbid psychiatric conditions is presented in Supplementary Table 1, and data on ASPD subjects’ violent offenses are presented in Supplementary Table 2.
Participants were aged 18–49 years. None of the participants screened positive for drugs of abuse, including cannabis. Healthy participants (e.g., no ASPD, no SCZ) and community participants with SCZ had previously participated in other PET experiments36,37. Groups did not differ in terms of age, ethnicity, body mass index, or number of participants with the C385A FAAH genotype. The ASPD group had fewer years of education compared with the control group. Therefore, education level was included as a covariate in the multivariate analyses, as the necessity of matching or controlling for this variable, potentially as a proxy for IQ, has been previously identified in studies of antisocial populations38.
Comparison of [11C]CURB λk 3 in ASPD and control groups
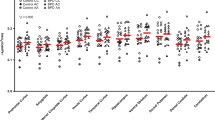
We observed an overall effect of ASPD diagnosis on [11C]CURB λk3 across the two hypothesized regions of interest (F2,26 = 4.5, p = 0.020). Using the effect of ASPD diagnosis in the MANCOVA for our two hypothesized ROIs (OFC and amygdala), results revealed that ASPD subjects had 12.5% lower amygdala [11C]CURB λk3 compared with the control subjects (0.12 ± 0.033 versus 0.15 ± 0.029, F1,27 = 7.2, p = 0.013). When we re-ran the analysis without incorporating education as a covariate, results remained significant (p = 0.046). However, there was no significant difference in OFC [11C]CURB λk3 between groups (11.7% reduction; 0.12 ± 0.028 versus 0.14 ± 0.021, F1,27 = 1.7, p = 0.21). There were no significant interactions. Genotype remained significant.
No significant effect of group was ascertained when we sampled the 11 ROIs for the whole-brain analysis (F11,17 = 1.4, p = 0.27; percentage of decreased FAAH binding in ASPD for each ROI: 10.6–12.5%). None of the regions survived Bonferroni correction. These results are presented graphically in Fig. 1. Statistics for secondary ROIs are presented in Supplementary Table 3. As expected, there was a main effect of genotype for the analysis (p = 0.003). There were no significant interactions.
Partial correlations between [11C]CURB λk 3 and hostility, aggression, impulsivity, psychopathic traits, and anxiety
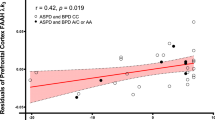
We found significant negative correlations between dorsal caudate (r = −0.58, p = 0.023), dorsal putamen (r = −0.55, p = 0.034), and cerebellum (r = −0.60, p = 0.017) [11C]CURB λk3 with sensation-seeking impulsivity, controlling for genotype, and a significant negative association between cerebellum [11C]CURB λk3 (r = −0.54, p = 0.035) and assaultive behavior, controlling for genotype. None of the partial correlations between the 11 ROI [11C]CURB λk3 values and reactive aggression, psychopathic traits, or anxiety were significant (all p-values > 0.05) (Figs. 2–5).
Conclusion
Consistent with our main hypothesis, we found that amygdala [11C]CURB λk3 was lower in ASPD versus participants without ASPD. We also discovered that sensation-seeking impulsivity in the ASPD group was negatively correlated with dorsal caudate, dorsal putamen, and cerebellum [11C]CURB λk3, while cerebellum [11C]CURB λk3 was negatively associated with assaultive behavior. To our knowledge, this PET study is the first to sample a target beyond the monoaminergic systems in ASPD. Although our sample is relatively small and the robustness of the statistical tests is modest, we believe that these findings can potentially provide a novel neurochemical mechanism to partially explain core deficits in ASPD related to reactive aggression and impulsivity.
We observed decreased amygdala [11C]CURB λk3. However, none of the other regions sampled had significantly lower [11C]CURB λk3 values. Lower amygdala FAAH expression is consistent with decreased FAAH expression observed across the brain in other externalizing conditions, such as alcohol and substance use disorders37,39. Our study was likely underpowered to detect differences in all ROIs. We suggest that alterations in [11C]CURB λk3 may be disorder-specific depending on whether internalizing or externalizing spectra are more prominent. For example, we would expect that primarily internalizing disorders, including major depressive disorder or posttraumatic stress disorder, would display higher brain FAAH density. Conducting [11C]CURB PET experiments of FAAH density in these and other psychiatric disorders could help answer this question.
Mounting evidence points to the role of the amygdala in stress-related psychiatric disorders and how the amygdala exhibits plasticity in response to acute stressors. This line of inquiry has implications for understanding the mechanisms of reactive aggression in ASPD. Interestingly, administration of FAAH inhibitors to animal models prevents morphological changes of the amygdala typically observed following stressful insults40, which might help explain the impaired stress response and propensity to respond with reactive aggression in ASPD. As a result, we suggest that lower levels of amygdala FAAH may impair how ASPD individuals process negative emotional stimuli, such as fearful or angry facial expressions. Mechanistically, lower FAAH levels should lead to greater CB1 neurotransmission, which may be responsible for hyper-reactivity of the amygdala in response to stressful, threatening situations. We speculate that lower levels of amygdala FAAH expression may prevent persons with ASPD from responding appropriately to stress, making them more inclined to engage in reactive aggressive and impulsive behavior.
A second main finding is that assaultive aggression was negatively correlated with cerebellum [11C]CURB λk3. We do note, however, that the cerebellum was not an a priori hypothesized ROI. Alterations in cerebellum structure and function have been previously reported in violent offenders41,42,43,44. Moreover, converging lines of neuroimaging evidence link the cerebellum to moral behavior and aggression45. Interestingly, the cerebellum has very high CB1 density46,47, and in the cerebellum, FAAH-positive cell bodies abut axon terminals containing CB1 receptors48. Thus, the ECS is highly expressed in cerebellum and contributes to cerebellar function49,50. There is elevated aggressive behavior in CB1 knockout mice51, and cannabinoid agonists are being investigated in clinical trials for treating agitation and aggression in Alzheimer’s patients52. Still, little is known about the role of cerebellar ECS in aggressive behavior. Hence, there is a need for more investigation to better understand the relationship between cerebellar FAAH and aggressive behavior.
The negative correlations reported between striatal/cerebellar FAAH binding and impulsivity mirror previous results of lower FAAH binding and higher trait impulsivity among individuals with cannabis use disorder during early abstinence39. There is high heritability of impulsivity in externalizing conditions, such as substance use disorders and ASPD53. Thus, genes regulating FAAH availability may be particularly important for impulsive phenotypes in externalizing conditions. Sensation-seeking impulsivity, in particular, is elevated among individuals who perpetrate antisocial behavior54. The neural substrate of impulsivity includes dysfunctional fronto-cerebellar connections55 and faulty fronto-striatal networks, consistent with the regions identified in the current study56. Since animal work suggests that FAAH deficiency increases motivations for rewards and sensation seeking57, low FAAH expression in these regions may predispose to impulsivity among individuals with high genetic predisposition for this trait.
There are currently no FDA-approved psychotherapeutics for the treatment of ASPD. Moreover, typically applied agents, such as antidepressants, mood stabilizers, anticonvulsants, and antipsychotics, have not been subject to rigorous testing. Since our preliminary data tentatively indicate that low FAAH may be implicated in the pathophysiology of ASPD, impulsivity, and aggression, interventions capable of increasing FAAH activity in a regionally-specific manner could possibly emerge as viable treatment options for ASPD. Consistent with this notion, leptin is a peripheral, adipose-derived hormone that functions as an indicator to the central nervous system (CNS) of energy storage58. There are several isoforms of the leptin receptor, which curb caloric intake, and which are widely distributed in the CNS. For example, the long form of the leptin receptor is expressed in Purkinje cells and dentate nucleus of the cerebellum as well as the amygdala among other regions in the human brain59. Leptin has recently been shown to increase brain FAAH levels in a knock-in mouse model of low FAAH activity (C385A polymorphism, rs324420)60. Similar to claims that reinstating FAAH activity to suppress AEA activity could underlie new anti-obesity treatments60, these same postulated therapeutics may also be effective for reducing reactive aggression and impulsivity. Testing such therapeutics on individuals with ASPD could open up new avenues for treatment.
We recently reported that there were no significant group differences between amygdala FAAH expression in borderline personality disorder (BPD) and healthy controls21. Given some similarities in clinical characteristics between BPD and ASPD, the contrasting effects could suggest at least two interpretations. One possibility is that one of the findings is spurious. A second possibility is that we have identified a brain feature that may account for differences between BPD and ASPD. We favor the latter interpretation, as molecular differences have previously been reported in the two conditions. For example, in one PET study, brain density of monoamine oxidase-A (MAO-A), an enzyme that degrades catecholamine neurotransmitters in the CNS, was greater in the PFC of BPD compared with healthy controls61. MAO-A total distribution volume also correlated with depression and suicidality in the BPD participants. Conversely, in another PET study of ASPD that also assayed brain MAO-A total distribution volume, orbitofrontal MAO-A levels were lower in ASPD compared with a control group composed of healthy individuals and some with alcohol dependence62. Therefore, we tentatively suggest that differential amygdala FAAH expression in ASPD and BPD could reflect a biomarker that is diagnosis-specific.
We note several limitations of the present investigation. First, the study design cannot delineate whether lower [11C]CURB λk3 in ASPD is a state or trait marker of psychopathology. Longitudinal designs that measured amygdala [11C]CURB λk3 at several time points could determine whether this biomarker is malleable to clinical improvement. Second, we acknowledge that our sample size is relatively small. Given some evidence that antisocial populations with and without comorbid anxiety disorders may manifest different brain correlates63, future PET studies with larger samples may consider tackling the question of whether amygdala [11C]CURB λk3 differs according to the presence or absence of anxiety disorders in violent offenders. Third, we did not measure serum levels of endocannabinoids such as AEA or the N-acylethanolamides N-palmitoylethanolamine and N-oleoylethanolamine. While measuring plasma concentrations of FAAH substrates could potentially identify peripheral biomarkers of brain FAAH activity, the relationship between brain and peripheral concentration of ECs is currently obscured due to excess physiological noise64. Fourth, we were unable to match participants from each group on the exact FAAH polymorphism (rs324420), although we found no difference in the proportion between groups and we also controlled for genotype. While the frequency distribution of the C385A genotype in ASPD is unknown in the general population, preliminary evidence suggests that it could be higher in individuals with ASPD14. Fifth, although we found group differences in the a priori ROI (amygdala), the correlations were detected in other brain regions. We draw attention to this fact, as these findings would not meet statistical significance under most corrections. Thus, these results should be viewed with caution. Sixth, although there is a modest positive correlation between intelligence test scores and academic achievement (data which we had available)65,66, we did not have IQ scores for all participants. We also found group differences in educational achievement; it is conceivable that group differences may also have been observed if IQ data had been available for all subjects. Future studies should endeavor to include IQ as a measure in PET studies of antisocial offenders, given that components of the endocannabinoid system may be linked to IQ, as has been shown for serotonin levels and cognition67,68. Finally, our sample of ASPD participants were all convicted of their violent crimes and, therefore, may differ in important ways from other ASPD individuals who offend but successfully evade the law.
In conclusion, ASPD is a very common yet understudied condition whose pathological features may be amenable to novel psychotherapeutics. PET investigations of ASPD with novel radioligands are, therefore, critical to advance the field. We found that amygdala FAAH brain activity was lower in ASPD, a finding that resonates with previous studies of externalizing populations. Higher impulsivity and greater aggression were additionally associated with lower FAAH [11C]CURB λk3 in a region-specific manner. However, these results must be viewed with caution, given the small sample size and modest results.
References
Raine, A. Antisocial personality as a neurodevelopmental disorder. Annu. Rev. Clin. Psychol. 14, 259–289 (2018).
Robins, L. N. & Regier, D. A. (eds) Psychiatric Disorders in America: The Epidemiologic Catchment Area Study (The Free Press, New York, 1991).
Samuels, J. et al. Personality dimensions and criminal arrest. Compr. Psychiatry 45, 275–280 (2004).
Coid, J. et al. Violence and psychiatric morbidity in the national household population of Britain: public health implications. Br. J. Psychiatry 189, 12–19 (2006).
Moran, P. & Hodgins, S. The correlates of comorbid antisocial personality disorder in schizophrenia. Schizophr. Bull. 30, 791–802 (2004).
Blair, R. J. R. The neurobiology of impulsive aggression. J. Child Adolesc. Psychopharmacol. 26, 4–9 (2016).
Nelson, R. J. & Trainor, B. C. Neural mechanisms of aggression. Nat. Rev. Neurosci. 8, 536–546 (2007).
Coccaro, E. F., McCloskey, M. S., Fitzgerald, D. A. & Phan, K. L. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry 62, 168–178 (2007).
Lee, T. M., Chan, S. C. & Raine, A. Strong limbic and weak frontal activation to aggressive stimuli in spouse abusers. Mol. Psychiatry 13, 655–656 (2008).
Siep, N. et al. Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging Behav. 13, 1311–1323 (2019).
Winstanley, C. A., Theobald, D. E., Cardinal, R. N. & Robbins, T. W. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J. Neurosci. 24, 4718–4722 (2004).
Kolla, N. J. & Houle, S. Single-photon emission computed tomography and positron emission tomography studies of antisocial personality disorder and aggression: a targeted review. Curr. Psychiatry Rep. https://doi.org/10.1007/s11920-019-1011-6 (2019).
McCluskey, S. P., Plisson, C., Rabiner, E. A. & Howes, O. Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur. J. Nucl. Med. Mol. Imaging 47, 451–489 (2019).
Hoenicka, J. et al. Association in alcoholic patients between psychopathic traits and the additive effect of allelic forms of the CNR1 and FAAH endocannabinoid genes, and the 3 ‘ region of the DRD2 gene. Neurotox. Res. 11, 51–59 (2007).
Kolla, N. J. & Mishra, A. The endocannabinoid system, aggression, and the violence of synthetic cannabinoid use, borderline personality disorder, antisocial personality disorder, and other psychiatric disorders. Front. Behav. Neurosci. https://doi.org/10.3389/fnbeh.2018.00041 (2018).
Zanettini, C. et al. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. https://doi.org/10.3389/fnbeh.2011.00057 (2011).
Di Marzo, V. Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 7, 438–455 (2008).
Kano, M., Ohno-Shosaku, T., Hashimotodani, Y., Uchigashima, M. & Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380 (2009).
Vinod, K. Y. et al. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J. Psychiatr. Res. 44, 591–597 (2010).
Boileau, I. et al. The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [11C]CURB. J. Cereb. Blood Flow. Metab. 35, 1237–1240 (2015).
Kolla, N. J. et al. Elevated fatty acid amide hydrolase in the prefrontal cortex of borderline personality disorder: a [(11)C]CURB positron emission tomography study. Neuropsychopharmacology 45, 1834–1841 (2020).
Yang, Y. & Raine, A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 174, 81–88 (2009).
Baldinger-Melich, P. et al. The effect of electroconvulsive therapy on cerebral monoamine oxidase A expression. Brain Stimul. 12, 714–723 (2019).
First, M. B., Gibbon, M., Spitzer, R. L., Williams, J. B. W. & Benjamin, L. S. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (American Psychiatric Press, Inc., Washington, 1997).
First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P), Version 2 (Biometrics Research, New York State Psychiatric Institute, New York, 2002).
Rusjan, P. M. et al. Mapping human brain fatty acid amide hydrolase activity with PET. J. Cereb. Blood Flow. Metab. 33, 407–414 (2013).
Boileau, I. et al. Whole-body radiation dosimetry of 11C-carbonyl-URB694: a PET tracer for fatty acid amide hydrolase. J. Nucl. Med. 55, 1993–1997 (2014).
Mizrahi, R. et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J. Cereb. Blood Flow. Metab. 32, 968–972 (2012).
Rusjan, P. et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 147, 79–89 (2006).
Whiteside, S. P. & Lynam, D. R. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers. Indiv. Differ. 30, 669–689 (2001).
Buss, A. H. & Durkee, A. An inventory for assessing different kinds of hostility. J. Consult. Psychol. 21, 343–349 (1957).
Raine, A. et al. The Reactive-proactive aggression questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggress. Behav. 32, 159–171 (2006).
Hart, S. D., Cox, D. N. & Hare, R. D. Mannual for the Hare Psychopathy Checklist: Screening Version (Multi-Health Systems, Toronto, 1995).
Patrick, C. J., Fowles, D. C. & Krueger, R. F. Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Dev. Psychopathol. 21, 913–938 (2009).
Spielberger, C. D., Gorsuch, R. L., Lushene, P. R., Vagg, P. R. & Jacobs, G. A. Manual for the State-trait Anxiety Inventory (form Y) (Consulting Psychologists Press, Palo Alto, 1983).
Watts, J. J. et al. Imaging brain fatty acid amide hydrolase in untreated patients with psychosis. Biol. Psychiatry 88, 727–735 (2020).
Best, L. M. et al. Lower brain fatty acid amide hydrolase in treatment-seeking patients with alcohol use disorder: a positron emission tomography study with [C-11] CURB.Neuropsychopharmacology 45, 1289–1296 (2020).
da Cunha-Bang, S. et al. Serotonin 1B receptor binding is associated with trait anger and level of psychopathy in violent offenders. Biol. Psychiatry 82, 267–274 (2017).
Boileau, I. et al. Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [(11)C]CURB. Biol. Psychiatry 80, 691–701 (2016).
Yasmin, F. et al. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc. Natl Acad. Sci. USA 117, 650–655 (2020).
Tiihonen, J. et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Res. 163, 201–212 (2008).
Leutgeb, V. et al. Altered cerebellar-amygdala connectivity in violent offenders: a resting-state fMRI study. Neurosci. Lett. 610, 160–164 (2016).
Leutgeb, V. et al. Brain abnormalities in high-risk violent offenders and their association with psychopathic traits and criminal recidivism. Neuroscience 308, 194–201 (2015).
Kolla, N. J., Gregory, S., Attard, S., Blackwood, N. & Hodgins, S. Disentangling possible effects of childhood physical abuse on gray matter changes in violent offenders with psychopathy. Psychiatry Res. 221, 123–126 (2013).
Demirtas-Tatlidede, A. & Schmahmann, J. D. Morality: incomplete without the cerebellum? Brain https://doi.org/10.1093/brain/awt070 (2013).
Herkenham, M. et al. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. USA 87, 1932–1936 (1990).
Mailleux, P. & Vanderhaeghen, J. J. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48, 655–668 (1992).
Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884 (2003).
Safo, P. K. & Regehr, W. G. Endocannabinoids control the induction of cerebellar LTD. Neuron 48, 647–659 (2005).
Kreitzer, A. C., Carter, A. G. & Regehr, W. G. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron 34, 787–796 (2002).
Rodriguez-Arias, M. et al. CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology 75, 172–180 (2013).
Herrmann, N. et al. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am. J. Geriatr. Psychiatry 27, 1161–1173 (2019).
Niv, S., Tuvblad, C., Raine, A., Wang, P. & Baker, L. A. Heritability and longitudinal stability of impulsivity in adolescence. Behav. Genet. 42, 378–392 (2012).
Mann, F. D. et al. Sensation seeking and impulsive traits as personality endophenotypes for antisocial behavior: evidence from two independent samples. Pers. Individ. Dif. 105, 30–39 (2017).
Miquel, M., Nicola, S. M., Gil-Miravet, I., Guarque-Chabrera, J. & Sanchez-Hernandez, A. A. Working hypothesis for the role of the cerebellum in impulsivity and compulsivity. Front. Behav. Neurosci. https://doi.org/10.3389/fnbeh.2019.00099 (2019).
Ersche, K. D. et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134, 2013–2024 (2011).
Tourino, C., Oveisi, F., Lockney, J., Piomelli, D. & Maldonado, R. FAAH deficiency promotes energy storage and enhances the motivation for food. Int. J. Obes. 34, 557–568 (2010).
Ahima, R. S. & Flier, J. S. Leptin. Annu Rev. Physiol. 62, 413–437 (2000).
Burguera, B. et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology 71, 187–195 (2000).
Balsevich, G. et al. Role for fatty acid amide hydrolase (FAAH) in the leptin-mediated effects on feeding and energy balance. Proc. Natl Acad. Sci. USA 115, 7605–7610 (2018).
Kolla, N. J. et al. Elevated monoamine oxidase-A distribution volume in borderline personality disorder is associated with severity across mood symptoms, suicidality, and cognition. Biol. Psychiatry 79, 117–126 (2016).
Kolla, N. J. et al. Lower monoamine oxidase-A total distribution volume in impulsive and violent male offenders with antisocial personality disorder and high psychopathic traits: an [(11)C] harmine positron emission tomography study. Neuropsychopharmacology 40, 2596–2603 (2015).
Sterzer, P., Stadler, C., Krebs, A., Kleinschmidt, A. & Poustka, F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol. Psychiatry 57, 7–15 (2005).
Hillard, C. J. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 43, 155–172 (2018).
Ritchie, S. J. & Tucker-Drob, E. M. How much does education improve intelligence? A meta-analysis. Psychol. Sci. 29, 1358–1369 (2018).
Rowe, D. C., Vesterdal, W. J. & Rodgers, J. L. Herrnstein’s syllogism: genetic and shared environmental influences on IQ, education, and income. Intelligence 26, 405–423 (1999).
Haahr, M. E. et al. The 5-HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum. Brain Mapp. 34, 3066–3074 (2013).
Penttila, J. et al. Verbal memory and 5-HT1A receptors in healthy volunteers—a PET study with [carbonyl-11C] WAY-100635. Neuropsychopharmacology 26, 570–577 (2016).
Funding
This study was funded by a Canadian Institutes of Health Research (CIHR) Project Grant and a CIHR Clinician Scientist Salary Award both awarded to NJK.
Author information
Authors and Affiliations
Contributions
N.J.K. was responsible for conception of the work and the acquisition, analysis, and interpretation of the data for the work. He also drafted the work, revised it for intellectual content, and gave final approval of the version to be published. I.B. interpreted the data for the work and helped revise it for important intellectual contributions. She gave final approval of the version to be published. K.K. made substantial contributions to the acquisition of the data and helped revise it critically for intellectual content. She gave final approval of the version to be published. J.W. made substantial contributions to the acquisition of the data and helped revise it critically for intellectual content. He gave final approval of the version to be published. P.R. made substantial contributions to the conception of the work. He helped revise the manuscript for important intellectual content and gave final approval of the version to be published. S.H. made substantial contributions to the conception of the work. He helped revise the manuscript for important intellectual context and gave final approval of the version to be published. R.M. made substantial contributions to the conception of the work. She helped revise the manuscript for important intellectual context and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kolla, N.J., Boileau, I., Karas, K. et al. Lower amygdala fatty acid amide hydrolase in violent offenders with antisocial personality disorder: an [11C]CURB positron emission tomography study. Transl Psychiatry 11, 57 (2021). https://doi.org/10.1038/s41398-020-01144-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-020-01144-2
This article is cited by
-
Psychopathology of antisocial personality disorder: from the structural, functional and biochemical perspectives
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)
-
Neural correlates of aggression in personality disorders from the perspective of DSM-5 maladaptive traits: a systematic review
Translational Psychiatry (2023)
-
Higher trait neuroticism is associated with greater fatty acid amide hydrolase binding in borderline and antisocial personality disorders
Scientific Reports (2022)