Minimization of N2O Emission through Intermittent Aeration in a Sequencing Batch Reactor (SBR): Main Behavior and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
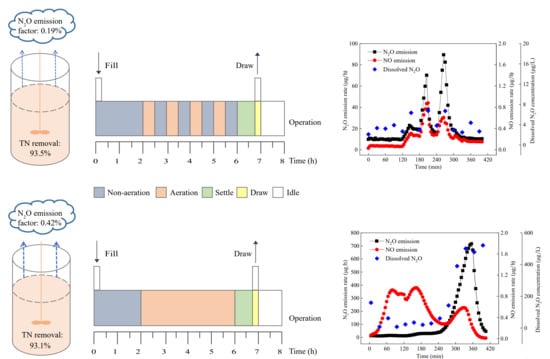
2.1. Reactor Setup and Operation
2.2. Batch Experiments for N2O Generation Mechanisms
2.3. Quantification of Comammox, AOB and AOA
2.4. Analytical Methods
3. Results and Discussion
3.1. Nitrogen Removal and N2O Emission in the Two SBRs
3.2. Nitrogen Removal and N2O Emission in Typical Cycles of the SBRs
3.3. Mechanisms of N2O Emission From Both Reactors
3.4. Potential Roles of Comammox Bacteria in N2O Mitigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, T.; Abbasi, T.; Luithui, C.; Abbasi, S.A. Modelling Methane and Nitrous Oxide Emissions from Rice Paddy Wetlands in India Using Artificial Neural Networks (ANNs). Water 2019, 11, 2169. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Climate change 2007 Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, UK; New York, NY, USA, 2007; p. 996. [Google Scholar]
- Rodriguez-Caballero, A.; Aymerich, I.; Marqués, R.; Poch, M.; Pijuan, M. Minimizing N2O emissions and carbon footprint on a full-scale activated sludge sequencing batch reactor. Water Res. 2015, 71, 1–10. [Google Scholar] [CrossRef]
- Akella, C.; Bhallamudi, S.M. Managing Municipal Wastewater Treatment to Control Nitrous Oxide Emissions from Tidal Rivers. Water 2019, 11, 1255. [Google Scholar] [CrossRef] [Green Version]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
- Desloover, J.; E Vlaeminck, S.; Clauwaert, P.; Verstraete, W.; Boon, N. Strategies to mitigate N2O emissions from biological nitrogen removal systems. Curr. Opin. Biotechnol. 2012, 23, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.; Van Loosdrecht, M.C. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, L.; Wang, D.; Zhou, Y.; Yang, X. Recent advances in nitrous oxide production and mitigation in wastewater treatment. Water Res. 2020, 184, 116168. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chandran, K. Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol. Bioeng. 2010, 106, 390–398. [Google Scholar] [CrossRef]
- Liu, D.-H.; Zhong, J.; Zheng, X.-L.; Fan, C.; Yu, J.; Zhong, W. N2O Fluxes and Rates of Nitrification and Denitrification at the Sediment–Water Interface in Taihu Lake, China. Water 2018, 10, 911. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Tian, Y.; Liu, T.; Ma, T.; Li, L.; Li, C.; Ahmad, M.; Chen, Q.; Ni, J. Minimization of nitrous oxide emission in a pilot-scale oxidation ditch: Generation, spatial variation and microbial interpretation. Bioresour. Technol. 2015, 179, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhou, N.; Liu, S.; Dang, C.; Liu, Y.; He, S.; Zhao, Y.; Liu, W.; Wanga, X. N2O and NO emission from a biological aerated filter treating coking wastewater: Main source and microbial community. J. Clean. Prod. 2019, 213, 365–374. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Camp, H.J.M.O.D.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Wang, M.; Zhao, Z.; Zhou, N.; He, S.; Liu, S.; Wang, J.; Wanga, X. Transcriptional activity and diversity of comammox bacteria as a previously overlooked ammonia oxidizing prokaryote in full-scale wastewater treatment plants. Sci. Total Environ. 2019, 656, 717–722. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Su, Q.; Mark, J.M.; Domingo-Félez, C.; Kiil, A.S.; Thamdrup, B.; Jensen, M.M.; Smets, B.F. Low nitrous oxide production through nitrifier-denitrification in intermittent-feed high-rate nitritation reactors. Water Res. 2017, 123, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Zhou, N.; He, S.; Chang, F.; Zhong, J.; Xu, S.; Wang, Z.; Liu, T. Nitrous oxide (N2O) emissions from a pilot-scale oxidation ditch under different COD/N ratios, aeration rates and two shock-load conditions. J. Environ. Manag. 2020, 111657, 111657. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, J.; Wang, S.-G.; Tan, L.-R.; Luo, J.-N.; Tao, Z.-Y.; Wang, S.-G. Effects of the Food-to-Microorganism (F/M) Ratio on N2O Emissions in Aerobic Granular Sludge Sequencing Batch Airlift Reactors. Water 2017, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Fujiwara, T.; Matsukawa, K.; Funamizu, N. Nitrous oxide emission mechanisms during intermittently aerated composting of cattle manure. Bioresour. Technol. 2013, 141, 205–211. [Google Scholar] [CrossRef]
- Yu, R.; Kampschreur, M.J.; Van Loosdrecht, M.C.; Chandran, K. Mechanisms and Specific Directionality of Autotrophic Nitrous Oxide and Nitric Oxide Generation during Transient Anoxia. Environ. Sci. Technol. 2010, 44, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-J.; Smets, B.F.; Yuan, Z.; I Nàcher, C.P. Model-based evaluation of the role of Anammox on nitric oxide and nitrous oxide productions in membrane aerated biofilm reactor. J. Membr. Sci. 2013, 446, 332–340. [Google Scholar] [CrossRef]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitrification, denitrification and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.J.; Shuster, W.D.; Rebholz, J.A. Nitrous Oxide Emissions from a Large, Impounded River: The Ohio River. Environ. Sci. Technol. 2010, 44, 7527–7533. [Google Scholar] [CrossRef] [PubMed]
- Tallec, G.; Garnier, J.; Billen, G.; Gousailles, M. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: Effect of oxygenation level. Water Res. 2006, 40, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, G.; Zhao, Z.; Dang, C.; Liu, W.; Zheng, M. Newly designed primer pair revealed dominant and diverse comammox amoA gene in full-scale wastewater treatment plants. Bioresour. Technol. 2018, 270, 580–587. [Google Scholar] [CrossRef]
- Zeng, R.J.; Lemaire, R.; Yuan, Z. Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor. Biotechnol. Bioeng. 2003, 84, 170–178. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Ma, B.; Li, J.; Ma, L.; Wang, S.; Li, J. Rapid Achievement of Nitritation Using Aerobic Starvation. Environ. Sci. Technol. 2017, 51, 4001–4008. [Google Scholar] [CrossRef]
- Ge, S.; Peng, Y.; Qiu, S.; Zhu, A.; Ren, N. Complete nitrogen removal from municipal wastewater via partial nitrification by appropriately alternating anoxic/aerobic conditions in a continuous plug-flow step feed process. Water Res. 2014, 55, 95–105. [Google Scholar] [CrossRef]
- Pan, M.; Chen, T.; Hu, Z.; Zhan, X. Assessment of nitrogen and phosphorus removal in an intermittently aerated sequencing batch reactor (IASBR) and a sequencing batch reactor (SBR). Water Sci. Technol. 2013, 68, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kornaros, M.; Dokianakis, S.N.; Lyberatos, G. Partial Nitrification/Denitrification Can Be Attributed to the Slow Response of Nitrite Oxidizing Bacteria to Periodic Anoxic Disturbances. Environ. Sci. Technol. 2010, 44, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lawlor, P.G.; Li, J.; Zhan, X. Characteristics of Nitrous Oxide (N2O) Emissions from Intermittently-Aerated Sequencing Batch Reactors Treating the Separated Liquid Fraction of Anaerobically Digested Pig Manure. Water Air Soil Pollut. 2011, 223, 1973–1981. [Google Scholar] [CrossRef]
- Zhang, F.; Li, P.; Chen, M.; Wu, J.; Zhu, N.; Wu, P.; Chiang, P.; Hu, Z. Effect of operational modes on nitrogen removal and nitrous oxide emission in the process of simultaneous nitrification and denitrification. Chem. Eng. J. 2015, 280, 549–557. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, J.; Miao, M.; Tian, L.; Guo, N.; Liang, S. Partial nitrification and nitrous oxide emission in an intermittently aerated sequencing batch biofilm reactor. Chem. Eng. J. 2013, 217, 435–441. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Wu, G.; Guan, Y. Nitrogen removal and nitrous oxide emission from a step-feeding multiple anoxic and aerobic process. Environ. Technol. 2017, 39, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, Y.; Pan, M.; Wu, G. Aerobic N2O emission for activated sludge acclimated under different aeration rates in the multiple anoxic and aerobic process. J. Environ. Sci. 2016, 43, 70–79. [Google Scholar] [CrossRef]
- Pan, M.; Wen, X.; Wu, G.; Zhang, M.; Zhan, X. Characteristics of nitrous oxide (N2O) emission from intermittently aerated sequencing batch reactors (IASBRs) treating slaughterhouse wastewater at low temperature. Biochem. Eng. J. 2014, 86, 62–68. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, Y.; Pan, M.; Zhan, X.; Hu, Z.; Wu, G. Enhanced biological nitrogen removal and N2O emission characteristics of the intermittent aeration activated sludge process. Rev. Environ. Sci. Biotechnol. 2017, 16, 761–780. [Google Scholar] [CrossRef]
- Cruz-Bournazou, M.N.; Hooshiar, K.; Arellano-Garcia, H.; Wozny, G.; Lyberatos, G. Model based optimization of the intermittent aeration profile for SBRs under partial nitrification. Water Res. 2013, 47, 3399–3410. [Google Scholar] [CrossRef]
- Salem, S.; Moussa, M.; Van Loosdrecht, M.C. Determination of the decay rate of nitrifying bacteria. Biotechnol. Bioeng. 2006, 94, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F.; Loeffler, B.; Polerecky, L.; Kuypers, M.M.M.; De Beer, D. Mechanisms of transient nitric oxide and nitrous oxide production in a complex biofilm. ISME J 2009, 3, 1301–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Chen, Q.; Ma, T.; Wang, M.; Ni, J. Genomic insights into metabolic potentials of two simultaneous aerobic denitrification and phosphorus removal bacteria, Achromobacter sp. GAD3 and Agrobacterium sp. LAD9. FEMS Microbiol. Ecol. 2018, 94, fiy020. [Google Scholar] [CrossRef] [PubMed]
- Conthe, M.; Lycus, P.; Arntzen, M.Ø.; Da Silva, A.R.; Frostegård, Å.; Bakken, L.R.; Kleerebezem, R.; Van Loosdrecht, M.C. Denitrification as an N2O sink. Water Res. 2019, 151, 381–387. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F. Nitrogen removal via short-cut simultaneous nitrification and denitrification in an intermittently aerated moving bed membrane bioreactor. J. Hazard. Mater. 2011, 195, 318–323. [Google Scholar] [CrossRef]
- Ribera-Guardia, A.; Marques, R.; Arangio, C.; Carvalheira, M.; Oehmen, A.; Pijuan, M. Distinctive denitrifying capabilities lead to differences in N2O production by denitrifying polyphosphate accumulating organisms and denitrifying glycogen accumulating organisms. Bioresour. Technol. 2016, 219, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lim, M.; Harjono, S.; Ng, W.J. Nitrous oxide emission by denitrifying phosphorus removal culture using polyhydroxyalkanoates as carbon source. J. Environ. Sci. 2012, 24, 1616–1623. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Tan, N.C.G.; Kleerebezem, R.; Picioreanu, C.; Jetten, M.S.M.; Van Loosdrecht, M.C.M. Effect of Dynamic Process Conditions on Nitrogen Oxides Emission from a Nitrifying Culture. Environ. Sci. Technol. 2008, 42, 429–435. [Google Scholar] [CrossRef]
- Caranto, J.D.; Vilbert, A.C.; Lancaster, K.M. Nitrosomonas europaeacytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc. Natl. Acad. Sci. USA 2016, 113, 14704–14709. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Liang, S.; Zhang, J.; Ngo, H.H.; Guo, W.; Yan, Y.; Zou, Y. Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: Sources and mechanisms. Bioresour. Technol. 2013, 136, 444–451. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- E Lawson, C.; Lücker, S. Complete ammonia oxidation: An important control on nitrification in engineered ecosystems? Curr. Opin. Biotechnol. 2018, 50, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Palomo, A.; Pedersen, A.G.; Fowler, S.J.; Dechesne, A.; Sicheritz-Pontén, T.; Smets, B.F. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J. 2018, 12, 1779–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camejo, P.Y.; Domingo, J.S.; McMahon, K.D.; Noguera, D.R. Genome-Enabled Insights into the Ecophysiology of the Comammox Bacterium “Candidatus Nitrospira nitrosa”. mSystems 2017, 2, e00059-17. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Han, P.; Hink, L.; Prosser, J.I.; Wagner, M.; Brüggemann, N. Abiotic Conversion of Extracellular NH2OH Contributes to N2O Emission during Ammonia Oxidation. Environ. Sci. Technol. 2017, 51, 13122–13132. [Google Scholar] [CrossRef] [Green Version]
- Kits, K.D.; Jung, M.-Y.; Vierheilig, J.; Pjevac, P.; Sedlacek, C.J.; Liu, S.; Herbold, C.; Stein, L.Y.; Richter, A.; Wissel, H.; et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Chen, L.; Wang, J.; Zheng, M.; Liu, S.; Chen, Q.; Ni, J. Comammox Nitrospira within the Yangtze River continuum: Community, biogeography, and ecological drivers. ISME J. 2020, 14, 2488–2504. [Google Scholar] [CrossRef]





| Configurations | Wastewater Types | N2O Emission Factor | References |
|---|---|---|---|
| Intermittently aerated SBR | Separated liquid fraction of anaerobically digested pig manure | 15.6% | [34] |
| Intermittently aerated SBR | Synthetic wastewater simulating the separated liquid fraction of anaerobically digested pig manure with a higher 5-day biochemical oxygen demand (BOD5) concentration | 10.1% | [34] |
| Sequencing batch air-lift reactor (SBAR) with anoxic-aerobic modes | Synthetic wastewater simulating municipal wastewater | 7.0 ± 1.6% | [35] |
| Intermittently aerated full-scale activated sludge SBR | Domestic wastewater | 6.8% | [4] |
| Intermittently aerated sequencing batch biofilm reactor (SBBR) | Synthetic ammonia-rich wastewater | 1.5 ± 0.2% | [36] |
| Step-feeding multiple A/O SBR | Synthetic wastewater simulating municipal wastewater | 4.4% | [37] |
| One-feeding multiple A/O SBR | Synthetic wastewater simulating municipal wastewater | 4.7% | [37] |
| Multiple A/O SBR | Synthetic wastewater simulating municipal wastewater | 2.3–10.1% | [38] |
| Intermittently aerated SBR | Slaughterhouse wastewater | 5.7–11.0% | [39] |
| Intermittently aerated SBR | Synthetic wastewater simulating municipal wastewater | 0.01–0.53% (0.19% on average) | This study |
| Experiment No. | Seed Sludge | Conditions | SBR1 | SBR2 | ||
|---|---|---|---|---|---|---|
| N2O/NO Emission Rates (μg h−1) | N2O/NO Emission Factors (%) | N2O/NO Emission Rates (μg h−1) | N2O/NO Emission Factors (%) | |||
| 1 | Anoxic phase | +nitrite a | 106.52/0.30 | 2.79/0.0079 | 280.83/0.33 | 11.41/0.0134 |
| 2 | Anoxic phase | +nitrite/+inhibitors b | 90.43/0.10 | 1.98/0.0034 | 305.69/0.08 | 8.65/0.0036 |
| 3 | Aerobic phase | +ammonia/+nitrite c | 281.02/0.41 | 6.47/0.0151 | 555.48/0.80 | 13.45/0.0308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, S.; He, S.; Tian, Z.; Zheng, M. Minimization of N2O Emission through Intermittent Aeration in a Sequencing Batch Reactor (SBR): Main Behavior and Mechanism. Water 2021, 13, 210. https://doi.org/10.3390/w13020210
Liu T, Liu S, He S, Tian Z, Zheng M. Minimization of N2O Emission through Intermittent Aeration in a Sequencing Batch Reactor (SBR): Main Behavior and Mechanism. Water. 2021; 13(2):210. https://doi.org/10.3390/w13020210
Chicago/Turabian StyleLiu, Tang, Shufeng Liu, Shishi He, Zhichao Tian, and Maosheng Zheng. 2021. "Minimization of N2O Emission through Intermittent Aeration in a Sequencing Batch Reactor (SBR): Main Behavior and Mechanism" Water 13, no. 2: 210. https://doi.org/10.3390/w13020210