Abstract
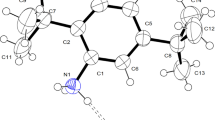
2-Chloro-N-(2,4-dinitrophenyl) acetamide, 1, was synthesized and characterized by 1H and 13C NMR spectroscopy, ESI–MS, X-ray crystallography, and elemental analysis. This compound crystallizes in the monoclinic space group P21/n. The crystal structure of compound 1 revealed the intramolecular H-bonding with the S(6) motif between H atom of the amide group and the nitro group at the ortho position. Several intermolecular C–H⋯O interactions hold different molecules of the compound 1 together resulting in the crystal packing. Red faint spots observed in the Hirshfeld surface of the compound 1 confirm the presence of N–H⋯O hydrogen bond as well as C–H⋯O interactions. According to the Hirshfeld surface, C–H⋯Cl interaction is also found, of which distance is relatively longer than the C–H⋯O distance. Moreover, the analysis of the corresponding fingerprint plots indicates the significant interactions within the crystal namely H⋯O/O⋯H (39.0%), C⋯O/O⋯C (10.6%), H⋯Cl/Cl⋯H (8.5%), H⋯H (7.3%), and H⋯C/C⋯H (5.9%) contacts. The optical properties of compound 1 in various solvents were investigated using UV–vis spectrophotometry. Compound 1 showed solvatochromic effects upon the varying polarity of the solvent. Time-dependent DFT calculations (TD-DFT) of compound 1 suggest that the deprotonation process occurs in polar solvents such as DMF.
Graphic Abstract
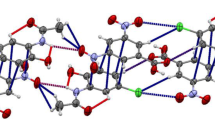
Crystal structure of 2-Chloro-N-(2,4-dinitrophenyl) acetamide (1) revealed the intramolecular N–H⋯O hydrogen bonding with the S(6) motif within the molecule as well as several intermolecular C–H⋯O interactions between molecules. Moreover, the compound 1 exhibited solvatochromic effects upon the varying polarity of the solvents.












Similar content being viewed by others
References
Burrows AD, Mingo DM, White AJP, Williams DM (1996) Chem Commun 97–99
Kolodiej HA, Orzecchowski K, Szostak R, Freundlich P, Glowiak T, Sorriso S (1996) J Mol Struct 380:15–22
Batsanov AS, Hubberstey P, Russell CE, Walton PH (1997) J Chem Soc Dalton Trans 15:2667–2672
Balaban AT (1997) J Am Chem Soc 119:11558–11566
Adamska EB, Jaskolski MJ, Brzezinski B (1999) J Mol Struct 475:167–173
An H, Li Y, Wang E, Xiao D, Sun C, Xu L (2005) Inorg Chem 44:6062–6070
M V, Kulkarni MV, Badami S, Yenagi J, Tonanavar J (2011) Spectrochim Acta Part A 84:137–143
Ozeryanskii VA, Pozharskii AF (2013) Tetrahedron 69:2107–2112
Fujio S, Hashizume D, Takamuki Y, Yasui M, Iwasaki F, Maki S, Niwa H, Ikeda H, Hirano T (2004) Tetrahedron Lett 45:8531–8534
Umeda R, Nishida H, Otono M, Nishiyama Y (2011) Tetrahedron Lett 52:5494–5496
Zimmermann LM, Nicolini J, Marini VG, Machado VG (2015) Sens Actuators B Chem 221:644–652
Lee DY, Singh N, Jang DO (2011) Tetrahedron Lett 52:1368–1371
Bagley MC, Lin Z, Pope SJA (2009) Tetrahedron Lett 50:6818–6822
Guerra JPTA, Lindner A, Nicoleti CR, Marini VG, Silva M, Machado VG (2015) Tetrahedron Lett 56:4733–4736
Qu Z, Li P, Zhang X, Wang E, Wang Y, Zhou P (2016) J Lumin 177:197–203
Luo M, Wang S, Wang M, Huang S, Li C, Chen L, Ma X (2016) Dyes Pigments 132:48–57
Singh AK, Singh RK (2015) J Mol Struct 1089:191–205
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Crystallogr 42:339–341
Sheldrick GM (2015) Acta Crystallogr A 71:3–8
Sheldrick GM (2015) Acta Crystallogr C 71:3–8
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, Platings M, Shields GP, Stevens JS, Towler M, Wood PA (2020) J Appl Crystallogr 53:226–235
Yanai T, Tew DP, Handy NC (2004) Chem Phys Lett 393:51–57
Frisch MJ et al (2009) Gaussian 09, revision B.01. Gaussian Inc., Wallingford
Cammi R, Tomasi J (1995) J Comput Chem 16:1449–1458
Zhao GJ, Han KL (2008) Phys Chem 9:1842
Fawcett WFJ (1993) Phys Chem 97:9540–9546
Andrews M, Ward BD, Laye RH, Kariuki BM, Pope SJA (2009) Helv Chim Acta 92:2159–2172
Gowda BT, Foro S, Fuess H (2007) Acta Crystallogr E 63:o1975
Zhang S-S, Xu L-L, Zou J, Bi S, Wen Y-H (2006) Acta Crystallogr E 62:o4478
Gowda BT, Kožíšek J, Tokarčík M, Fuess H (2008) Acta Crystallogr E 64:o987
Gowda BT, Foro S, Fuess H (2008) Acta Crystallogr E 64:o419
Gowda BT, Foro S, Fuess H (2008) Acta Crystallogr E 64:o420
Gowda BT, Foro S, Fuess H (2008) Acta Crystallogr E 64:o85
Mongkholkeaw S, Songsasen A, Duangthongyou T, Chainok K, Suramitr S, Wattanathana W, Wannalerse B (2020) Acta Crystallogr E 76:594–598
Hirshfeld FL (1977) Theor Chim Acta 44:129–138
Spackman MA, Jayatilaka D (2009) CrystEngComm 11:19–32
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) Crystal Explorer 17. The University of Western Australia, Perth
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Chem Commun 3814–3816
Hiroki M, Takumi O, Satoshi O (2016) Tetrahedron Lett 57:3011–3015
Sun Y, Wang G, Guo W (2009) Tetrahedron 65:3480–3485
Yin Z, Jiang L, He J, Cheng JP (2003) Chem Commun 2326–2327
Suramitr S, Hannongbua S, Wolschann P (2007) J Mol Struct (THEOCHEM) 807(1–3):109–119
Wattanathana W, Nootsuwan N, Veranitisagul C, Koonsaeng N, Suramitr S, Laobuthee A (2016) J Mol Struct 1109:201–208
Sriyab S, Gleeson MP, Hannongbua S, Suramitr S (2016) J Mol Struct 1125:532–539
Fawcett WR (1993) J Phys Chem 97:9540–9546
Catalán J, Palomar J, Díaz C, de Paz JLG (1997) J Phys Chem 101:5183–5189
Acknowledgements
This research is supported by the Thailand Research Fund (MRG 5580182), Kasetsart University Research and Development Institute, and Department of Chemistry, Kasetsart University, and the Center of Excellent for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jansukra, P., Wattanathana, W., Duangthongyou, T. et al. Synthesis, X-Ray Crystallography, Theoretical Investigation and Optical Properties of 2-Chloro-N-(2,4-dinitrophenyl) Acetamide. J Chem Crystallogr 51, 523–535 (2021). https://doi.org/10.1007/s10870-020-00875-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-020-00875-w