Abstract
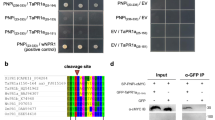
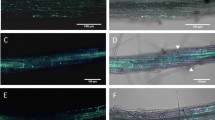
Rice sheath blight, a major devastating disease caused by Rhizoctonia solani, poses huge threat to global rice production. Despite being the most hostile necrotrophic fungus with a wide host range, the mechanism involved in R. solani pathobiology is poorly understood. The evolutionarily developed nonhost resistance (NHR) provides broad-spectrum disease resistance to food crops. According to previous reports, Arabidopsis PEN1, PEN2 and PEN3 act as key components of cell wall-based pre-haustorial defenses against non-adapted pathogens. However, the involvement of these PEN genes in NHR against R. solani has remained unexplored till date. Therefore, the objective of this work is to study the efficacy of Arabidopsis PEN genes in NHR against R. solani. For this, hyphal colonization and penetration structures were monitored in Arabidopsis wild type Columbia-0 (Col-0) and mutants- pen1, pen2–3 and pen3–1. Comparatively, R. solani colonization on the leaves of Arabidopsis wild types was less than that on the rice leaf surface. Also, among the pen mutants studied, pen2–3 allowed maximum penetration and colonization by R. solani during early hours of infection as evidenced by both microscopic and macroscopic observations. Advanced lesion area, reduced chlorophyll content and increased fungal biomass accumulation also corroborated with the disease severity in pen2–3. However, R. solani resistance was restored in complemented PEN2-GFP comparable to Col-0. Altogether, our results demonstrate major involvement of PEN2 during pre-penetration, and its contribution to NHR against R. solani for enhanced disease resistance.






Similar content being viewed by others
References
Ajayi-Oyetunde O, Bradley C (2018) Rhizoctonia solani: taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol 67:3–17
Azizi P, Rafii MY, Mahmood M, Abdullah SNA, Hanafi MM, Nejat N, Latif MA, Sahebi M (2015) Differential gene expression reflects morphological characteristics and physiological processes in rice immunity against blast pathogen. Magnaporthe oryzae PloS one 10:e0126188
Basu A, Chowdhury S, Ray Chaudhuri T, Kundu S (2016) Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant Pathol 65:1333–1346
Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Bittel P, Robatzek S (2007) Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr Opin Plant Biol 10:335–341
Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, Schulze-Lefert P (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425:973–977
Dodman R, Barker K, Walker J (1968) A detailed study of the different modes of penetration by Rhizoctonia solani. Phytopathology 58:1271–1276
Ellinger D et al. (2013) Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis Plant Physiology:pp 112.211011
Fauth M, Schweizer P, Buchala A, Markstädter C, Riederer M, Kato T, Kauss H (1998) Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2Elicitors plant physiology 117:1373-1380
Flentje N, Dodman R, Kerr A (1963) The mechanism of host penetration by Thanatephorus cucumeris. Aust J Biol Sci 16:784–799
Foley RC, Gleason CA, Anderson JP, Hamann T, Singh KB (2013) Genetic and genomic analysis of Rhizoctonia solani interactions with Arabidopsis; evidence of resistance mediated through NADPH oxidases PLoS one 8:e56814
Fuchs R, Kopischke M, Klapprodt C, Hause G, Meyer AJ, Schwarzländer M, Fricker MD, Lipka V (2016) Immobilized subpopulations of leaf epidermal mitochondria mediate PENETRATION2-dependent pathogen entry control in Arabidopsis. Plant Cell 28:130–145
Ghosh S, Kanwar P, Jha G (2017) Alterations in rice chloroplast integrity, photosynthesis and metabolome associated with pathogenesis of Rhizoctonia solani. Sci Rep 7:41610
Gill US, Lee S, Mysore KS (2015) Host versus nonhost resistance: distinct wars with similar arsenals. Phytopathology 105:580–587
Jones JD (2006) Dangl JL. The plant immune system Nature 444:323–329. https://doi.org/10.1038/nature05286
Kouzai Y et al. (2018) Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon new phytologist 217:771–783
Krattinger SG et al. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat science 323:1360-1363
Kumar KVK, Reddy M, Kloepper J, Lawrence K, Groth D, Miller M (2016) Sheath blight disease of rice (Oryza sativa L.)–an overview. Biosciences Biotechnology Research Asia 6:465–480
Kumar KVK et al (2011) Evaluation and selection of elite plant growth-promoting rhizobacteria for suppression of sheath blight of rice caused by Rhizoctonia solani in a detached leaf bio-assay. Int J Appl Biol Pharm Technol 2:488–495
Lazniewska J, Macioszek VK, Kononowicz AK (2012) Plant-fungus interface: the role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol Mol Plant Pathol 78:24–30
Li S, Peng X, Wang Y, Hua K, Xing F, Zheng Y, Liu W, Sun W, Wei S (2019) The effector AGLIP1 in Rhizoctonia solani AG1 IA triggers cell death in plants and promotes disease development through inhibiting PAMP-triggered immunity in Arabidopsis thaliana. Front Microbiol 10:2228
Lipka U, Fuchs R, Lipka V (2008) Arabidopsis non-host resistance to powdery mildews. Curr Opin Plant Biol 11:404–411
Lipka V et al. (2005) Pre-and postinvasion defenses both contribute to nonhost resistance in Arabidopsis science 310:1180-1183
Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol 123(3):795–806
Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J (2011) Callose deposition: a multifaceted plant defense response. Mol Plant-Microbe Interact 24:183–193
Maeda K, Houjyou Y, Komatsu T, Hori H, Kodaira T, Ishikawa A (2009) AGB1 and PMR5 contribute to PEN2-mediated preinvasion resistance to Magnaporthe oryzae in Arabidopsis thaliana. Mol Plant-Microbe Interact 22:1331–1340
Maeda K, Houjyou Y, Komatsu T, Hori H, Kodaira T, Ishikawa A (2010) Nonhost resistance to Magnaporthe oryzae in Arabidopsis thaliana. Plant Signal Behav 5:755–756
Mondal A, Dutta S, Nandi S, Das S, Chaudhuri S (2012) Changes in defence-related enzymes in rice responding to challenges by Rhizoctonia solani. Archives of phytopathology and plant protection 45:1840–1851
Mukherjee AK, Carp MJ, Zuchman R, Ziv T, Horwitz BA, Gepstein S (2010) Proteomics of the response of Arabidopsis thaliana to infection with Alternaria brassicicola. J Proteom 73(4):709–720
Nuernberger T, Lipka V (2005) Non-host resistance in plants: new insights into an old phenomenon. Mol Plant Pathol 6:335–345
Okorski A, Olszewski J, Pszczółkowska A, Kulik T (2008) Effect of fungal infection and the application of the biological agent EM 1TM on the rate of photosynthesis and transpiration in pea (Pisum sativum L.) leaves. Polish Journal of Natural Sciences 23:35–47
Pannecoucque J, Höfte M (2009) Interactions between cauliflower and Rhizoctonia anastomosis groups with different levels of aggressiveness BMC. Plant Biol 9:95
Park J-Y, Jin J, Lee Y-W, Kang S, Lee Y-H (2009) Rice blast fungus (Magnaporthe oryzae) infects Arabidopsis via a mechanism distinct from that required for the infection of rice. Plant Physiol 149:474–486
Rajalakshmi K, Banu N (2013) Extraction and estimation of chlorophyll from medicinal plants. International Journal of Science and Research:209–012
Sahu, BB, Sumit R, Srivastava SK, Bhattacharyya MK (2012) Sequence based polymorphic (SBP) marker technology for targeted genomic regions: its application in generating a molecular map of the Arabidopsis thaliana genome. BMC Genomics 13(1):20
Scalschi LM et al. (2015) Quantification of callose deposition in plant leaves
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Sharon M, Freeman S, Sneh B (2011) Assessment of resistance pathways induced in Arabidopsis thaliana by hypovirulent Rhizoctonia spp. isolates. Phytopathology 101:828–838
Singh A, Rohila R, WILLOCQUET SSL, SINGH U (2012) Infection process in sheath blight of rice caused by Rhizoctonia solani. Indian Phytopathology
Speth EB, Lee YN, He SY (2007) Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr Opin Plant Biol 10:580–586
Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18:731–746
Taheri P, Tarighi S (2011) Cytomolecular aspects of rice sheath blight caused by Rhizoctonia solani. Eur J Plant Pathol 129:511–528
Waetzig GH, Sobczak M, Grundler FM (1999) Localization of hydrogen peroxide during the defence response of Arabidopsis thaliana against the plant-parasitic nematode Heterodera glycines. Nematology 1:681–686
Wei M, Wang A, Liu Y, Ma L, Niu X, Zheng A (2020) Identification of the novel effector RsIA_NP8 in Rhizoctonia solani AG1 IA that induces cell death and triggers defense responses in non-host plants. Front Microbiol 11:1115
Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322:681–692
Zheng A, Lin R, Zhang D, Qin P, Xu L, Ai P, Ding L, Wang Y, Chen Y, Liu Y, Sun Z, Feng H, Liang X, Fu R, Tang C, Li Q, Zhang J, Xie Z, Deng Q, Li S, Wang S, Zhu J, Wang L, Liu H, Li P (2013) The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat Commun 4:1424
Acknowledgments
The authors thank Director, NIT Rourkela for providing all facilities at NIT Rourkela, Odisha INDIA. The authors also thank Dr. Arup K. Mukherjee, NRRI, Cuttack for providing the R. solani pure culture.
Financial support
Supported by the S & T Division, Govt. of Odisha (Grant number 27552800232014) and SERB, Govt. of India (YSS/2014/000142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors read the manuscript and declare that there is no conflict of interest.
Research involving human participants and/or animals
Not applicable. The research involved no human participants or animals.
Informed consent
The research involved no human participants, and no animals so that the statement on the welfare of animals is not required.
Supplementary Information
ESM 1
(PPTX 5868 kb)
Rights and permissions
About this article
Cite this article
Parween, D., Sultan, E., Dalei, K. et al. Arabidopsis nonhost resistance gene PENETRATION 2 is involved in disruption of cushion formation by Rhizoctonia solani during early infection process. Australasian Plant Pathol. 50, 281–292 (2021). https://doi.org/10.1007/s13313-020-00768-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00768-8