Abstract
Groundwater demand has increased due to overpopulation and intensive irrigation which resulted in decline of water resources and deterioration of water quality. In this view, a microlevel study was conducted through hydrogeochemical investigation and resistivity survey (VES) in Kalanad river basin, Kasaragod, Kerala. The study aims to determine the spatial variations in physicochemical parameters with delineation of geological layers of the study area through VES. Hydrogeochemical investigation and water suitability assessment for drinking and irrigation purposes were confined to open well water samples of the study area. The sample analysis and comparison with various standards (WHO and BIS) show that the groundwater is not entirely fit for drinking with respect to pH. Almost all samples fall within standard values as per the irrigation water quality indices. The chemical nature of the groundwater samples was dominated by alkalies and majority of the samples belonged to sodium chloride type. This study also dealt with the interpretation of hydrogeochemical data using correlation and R mode factor analyses. VES was carried out at seven locations, and the apparent resistivity values obtained had been analysed. The interpretation delineated a maximum of four geologic layers in the study area. The sequence of geologic formation is composed of highly resistive top lateritic soil followed by saturated zone and then the basement topography in the entire basin.
Similar content being viewed by others
Introduction
Water is the basis of life and it strikes on all themes of the agenda for development. The need for adequate fresh water resources is not only important for human existence, but also for the maintenance and sustainability of natural ecological systems. The quality of groundwater is as important as its quantity considering the suitability of water for various purposes (Kumar et al. 2009; Subramani et al. 2005). The industrial and agricultural pollutions due to anthropogenic disturbances, overconsumption and urbanization deteriorate the groundwater and make the water not suitable for any purposes (Carpenter et al. 1998; Jarvie et al.1998; Simeonov et al. 2003). Due to growing population, many parts of India are already facing acute water scarcity. India's water quality per capita has declined fourfold over the past 100 years, while it has declined fivefold for Kerala (Basak 1992). Even though the Kerala state receives copious amount of 3100 mm of rainfall annually which is 2.8 times of the national average, the per capita availability of freshwater in the state is one of the lowest among the other states of India.
Water chemistry is an important aspect to be considered prior to its use for domestic, irrigation and industrial purposes. Groundwater quality data cast light on the geological past and give clues on groundwater recharge, discharge and storage (Walton 1970). Several factors such as climate, soil, circulation of groundwater through rock types and saline water intrusion influence the quality of groundwater. In nature, the occurrence of chemically pure water for an appreciable length of time is not possible. Suitability of groundwater for domestic and irrigation purposes is determined by its groundwater geochemistry. The groundwater moves very slowly, compared to surface water, through the pore spaces in the surrounding country rocks. Thus, it gets more residence time and as a result of the interaction of the groundwater with the wall rocks, more ions will be dissolved into the groundwater. Unlike surface water bodies, if groundwater body is once contaminated, it will remain as it is for a long time (up to tens of hundreds of years) due to the very slow natural processing of the flushing. The suitability of natural water for a particular purpose is based on the criteria of acceptable quality for that use. Hence, the quality criteria for drinking water, industrial water and irrigation water differ widely. Numerous studies were attempted earlier to evaluate the ground water quality in various parts of India (Prasanna et al. 2011; Sreedevi 2004; Brinda et al. 2014; Balwant et al. 2016;). Exploration of groundwater and groundwater quality assessment by using geophysical techniques has been done widely. Electrical resistivity method is most commonly used in groundwater exploration. The resistivity methods can be used to estimate the thickness and electrical nature of the formation, which provides valuable knowledge about the groundwater potentialities (Griffith and King 1965; Parasins 1966 and Balakrishna1980). It also enables to identify and delineate fresh water and saline water zones.
The present study is carried out in a small watershed in the northern coastal region of Kerala state. The main objectives of the study are to assess the suitability of groundwater for domestic and irrigational purposes by means of hydrogeochemical analyses and to recognise the subsurface structure of the basin using geoelctrical surveying. Results of hydrogeochemical studies were used to assess the domestic and irrigational suitability of groundwater by comparing with various standards (BIS and WHO) and some parameters. The water chemistry data were also subjected to various analyses (Hill-Piper diagram and statistical analyses) to find out the intrinsic relationship among variables and hydrogeochemical characteristics of the study area.
Materials and methods
Study area
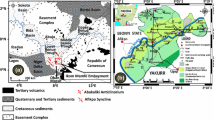
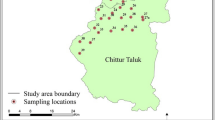
The study area is Kalanad river basin, and it falls within the western part of the Kasaragod district of Kerala state. It lies between longitude 74059′58.577″E to 75003′40.973″E and latitude 12025′37.315″N to 12028′55.128″N (Fig. 1). The river has a total length of 7.282 km, and the watershed has a total areal extend of 15.96 Sq.km. The crystalline rocks of Archaean age forms the basement rock and its exposures are limited to the shore line cliffs. Charnockite and gneiss are the major crystalline rocks present in the area in which gneiss is the major lithounit. Gneiss is of granitic composition and is foliated. Charnockites are seen as fractureless and non-foliated, and they represent granulite facies rocks of the area. Both of these crystalline rocks are extensively lateritised, and almost entire area is covered by laterite and alluvium. The tertiary sedimentary rocks of sandstone and clay with lignite intercalations and quaternary deposits of coastal sand and alluvium are the other major lithounits.
Groundwater chemistry and quality
Water quality is an important characteristic of any hydrogeological studies. The chemical quality of groundwater of an area depends on various parameters like geology, geomorphology, structural and agricultural activities (Vijayakumar et al. 2015). Properties such as amount of groundwater recharge, its mixing range, the circulation pathway, groundwater residence time and its temperature at depth can be evaluated using hydrochemical data (Edmunds 1994). In the present study, groundwater samples were carefully collected from the 16 representative open wells during January 2019. Water samples were collected in one litre capacity polyethene bottles which were pre cleaned, sterilized as per the standard sampling procedures. After carefully sealed and labelled, the samples were taken for analyses. The locations of water sample collection in the study area are shown in Fig. 1.The collected samples were analysed in the chemical laboratory, Department of Geology, University of Kerala. The parameters like electrical conductivity (EC) and hydrogen concentration (pH) were measured in the field at the time of sampling. All samples were chemically analysed according to the standard procedures prescribed by APHA (1998) and Trivedi and Goel (1986) for evaluating their chemical constituents. Using the standards specified by BIS (2012) and WHO (2004), the estimated values of the major cations, anions, pH, EC and TDS were compared. The estimation methods of each chemical constituent are given in the Table 1.
Irrigational quality of ground water
The suitability of water for irrigation purposes depends on numerous chemical constituents. Sodium adsorption ratio (SAR), residual sodium carbonate (RSC) and percent sodium are the common parameters for evaluating water quality for irrigation purposes. Using sodium adsorption ratio (SAR), the concentration of sodium Na+, Ca2+ and Mg2+ in the water samples can be studied. Excess sodium in water results in change of soil properties and reduction in soil permeability (Kelley 1951). Based on SAR values, water can be classified four classes such as excellent, good, fair and poor (Richards 1954).
Residual Sodium Carbonate is defined as
where the ionic concentration is in meq/L.
If carbonate and bicarbonate concentration exceeds the permissible limits, and is in excess compared to the alkaline earths mainly calcium and magnesium, it adversely affects agriculture (Eaton 1950, and Richards 1954). A negative RSC indicates that sodium build-up is not likely to occur whereas the positive RSC suggest that sodium build-up in the soil is possible. The water is unsuitable for irrigation if the RSC exceeds 2.5 meq/L, if RSC value range between 1.25 and 2.5 meq/L, then the water is of marginal quality, and the water is suitable for irrigation only if RSC is < 1.25 (Lloyd and Heathcote 1985). According to Richards (1954), irrigation water can be classified into three categories based on RSC values.
Percent sodium content is an important parameter to evaluate the suitability of water for agricultural purposes (Wilcox 1948). It is important because sodium reacts with the soil to reduce its permeability. For agricultural purposes, a maximum of 60% sodium in groundwater can be used (Ramakrishna 1998). Based on the percent sodium, Wilcox (1995) classified irrigation water into different categories such as ‘excellent’ (< 20 Na%), ‘good’ (20–40 Na%), ‘permissible’ (40–60 Na%), ‘doubtful’ (60–80 Na%) and ‘unsuitable’ (Na% > than 80%).
Hill-piper diagram
The trilinear diagram has been used extensively in understanding hydrochemical characteristic of groundwater. Trilinear diagram was first attempted by Hill (1940) and refined by Piper (1944) to describe water chemistry. Piper diagram was structured in such a pattern that milliequivalent percentages of cations and anions are plotted on the left triangle and the right triangle, respectively. Total cations and total anions are each considered as 100 percentages. To get the overall character of the water, these plotted points in the triangular fields are projected further into the central diamond field. To analyse the similarities and differences in the composition of water and to group them into different chemical types, the piper diagram has been widely used. Hydrochemical facies was extensively used in the chemical assessment of groundwater, and its concept is to identify the water composition in different classes. It provides information on the chemical quality of water and its origin. Back and co-workers (1965) suggested subdivisions of the trilinear diagram to define composition class.
Statistical analyses
For statistical analyses, hydrogeochemical data were analysed and interpreted using correlation and R mode factor analyses. Correlation analysis was performed in order to establish the associations among the various geochemical constituents. The end product of correlation analysis is correlation coefficient “r”, and it is a numerical value range between + 1 and − 1. A correlation coefficient r equal to + 1 indicates variable with a perfect linear relationship in a positive manner, the value of r equal to – 1 reflects a negative linear relationship among variables and condition where r equals zero, it reflects a nonlinear relationship between variables. Factor analysis was put in to understand the water chemistry characteristics that caused most of the variability within the dataset. It is a major tool to explain the similarities among variables. Extraction of the eigen values and the eigen vectors from the matrix of correlations or covariances is the process done in factor analysis (Davis 1973). A small number of factors can give the same amount of information as larger set of original observations since the factors reduces the overall complexity of the data by taking advantages of inherent interdependencies. Here R-mode factor analysis was done for hydrochemical data to reduce the number of variables. Principle component analysis is a statistical method where original data are changed to a form and can be evaluated in Euclidean space (Joliffe 2002). Varimax rotation is followed for principal component analysis, and the data interpretation was done by analysing the factors and its rotated loadings. Kaiser normalization consider only factor with eigen values greater than 1 for interpretation (Kaiser 1960).
Geoelectrical surveying
The applications of geophysical methods in the exploration of groundwater have been explained and reviewed by a number of workers (Zohdy et aI. 1974; Beeson and Jones 1988; Ward 1990; deStadelhofen 1994). One of the most important geophysical methods applied in groundwater exploration is geoelectrical prospecting. Electrical resistivity methods are widely employed for both regional and detailed surveys of groundwater exploration because of their greater resolving powers, low expense and wide range of field applicability (Chandra and Athavale 1979; Chandrasekhran 1988). Electrical resistivity technique is basically the response of the earth to flow of electric current. In general the depth of investigation of resistivity survey is directly proportional to the electrode spacing (Bhattacharya and Patra 1968; Zohdy et al. 1974; Koefoed 1979). The resistivity of a rock unit mainly depends on its mineral composition and interstitial water. Vertical electronic sounding (VES) is employed to obtain information of the layers of the subsurface. For the present study, seven soundings were carried out using Schlumberger configuration and analysed. In the surveys, the minimum and maximum values of current electrode separation (AB/2) are 2 to 90 m. Data of 7 VES of the study area were interpreted qualitatively and quantitatively to attain multi-layered resistivity profiles. The apparent resistivity and AB/2 values were plotted on double-log sheet in IPI2Win software (Moscow State University). Model for the layered resistivity is obtained in IPI2 Win, and it interprets different resistivity layers. The apparent resistivity, thickness and depth to layer interface were estimated from the layered resistivity model. Depth-wise isoresistivity maps were generated using QGIS for qualitative interpretation by using the apparent resistivity data at different depths obtained directly from VES.
Results and discussions
Suitability of water for domestic purposes
The results of the chemical analyses of water samples are given in Tables 2 and 3. These results were summarized and compared with the standard guideline values as recommended by the WHO(World Health Organization) and BIS (Bureau of Indian Standards) for drinking and public health purposes. The results show that all parameters fall within the permissible limits defined by BIS (2012) and WHO (2004) except pH. pH is the logarithm of the reciprocal of the hydrogen ion concentration, which expresses the scale of intensity of acidity or alkalinity of water. In the present study, pH of open well water samples varies from 5 to 6.68 with a mean of 5.78 which shows the general acidic trend of groundwater in the area. The spatial variation of pH is shown in Fig. 2 and it shows that more than 90% of the area is having a pH value of less than 6.
Suitability of water for irrigation
The chemistry and quality of groundwater plays a vital role in determining its use for agricultural purposes. The suitability of water for irrigation mainly depends on the response of plants and soil to the effects of mineral constituents in water (Hill 1942; Anon 1946). In addition to chemical quality of water, utilization of groundwater for irrigation purpose also relies on many factors such as climate, soil texture and composition type of crop and irrigation practices. The major parameters applied in the present study to determine the suitability of water for irrigation are sodium adsorption ratio (SAR), sodium percentage and residual sodium carbonate. SAR is the ratio between sodium with respect to calcium and magnesium (Iqbal et al. 2012; Haritash et al. 2014; Sridharan and Senthil Nathan 2017). According to the SAR values, the groundwater resources of the study area comes under the excellent category for irrigation purpose as all of the samples have SAR values less than 10 (Table 4). RSC index is generally used to establish the groundwater suitability for agricultural purposes in the case of clayey soil. The RSC values of the samples range from 0.176 meq/1 to − 5.03 meq/1. All the well samples are categorized under the good category of RSC values (Table 5). In the case of sodium percentage index, except location no.9, all other values mostly come in permissible range indicating that the open well water is good for irrigation purpose (Table 6). High content of sodium in irrigation water may bring about the exchange of sodium in water for calcium and magnesium ions, which will lead to the reduction in the internal drainage capacity of the soil (Collins and Jenkins 1996; Singh et al.2013).
Hill-piper diagram
A piper diagram was created using the analytical data from the study area. The diagram shows the intrinsic chemical relationship between the groundwater samples. Hydrochemical facies analysis is a valuable tool for establishing the chemical evolution of groundwater masses (Srinivasamoorthy et al. 2010). In majority of the samples (10 numbers), alkalies exceed the alkaline earths with respect to relative abundance of cations. Basically, the sample plots in the piper diagram can be classified into 6 fields such as Ca-HCO3 type, Na-Cl type, Ca–Mg–Cl type, Ca-Na-HCO3 type, Ca–Cl type and Na- HCO3 type (Fig. 3). In the study area, most of them are chloride type (13 samples), and the remaining three are bicarbonate type among the anionic species, which means strong acids exceeds exceed weak acids when considering the relative abundance of anions. Evaluation of the water types using piper plot suggests that nine samples are sodium chloride type (non-carbonate alkali exceeds 50%), six samples are confined to Mixed type (no cation and anion pairs exceeds 50%), and the remaining one belongs to Magnesium bicarbonate type (carbonate hardness exceeds 50%). The dominance of sodium ion in the water samples of the study area suggests the influence of nearby sea or the weathering of sodium bearing minerals or some cation exchange processes and agricultural activities.
Statistical analyses
In the present study, hydrogeochemical data were analysed and interpreted using correlation analysis and R mode factor analyses. Correlation analyses of the physicochemical parameters of the samples were carried out to understand the relationship between various parameters, and the analyses show strong correlations among some variables. The correlation matrix of the chemical parameters in the groundwater samples of the study area is shown in the Table 7. Electrical conductivity (EC) and total dissolved solids(TDS) have strong significant positive correlation with all other parameters except sulphate and nitrate. It reflects their discrete sources with respect to other ions. The source of sulphate and nitrate may be from fertilizers and biological origin, respectively. pH shows a strong correlation with alkalinity of bicarbonate type only. Total hardness indicates a stronger positive correlation with chloride when compared with bicarbonate which may indicate the dominant permanent hardness of the water.
Major components which account for 93% of total variance is extracted by R mode factor analysis (Table 8 and 9). Factor 1 attributes 72% of the variance which is characterised by very high loadings of EC, TDS, total hardness, calcium, magnesium, sodium, potassium and nitrate. It can be inferred that the factor 1 denotes TDS and permanent hardness of the water. The second factor which accounts for 12% of variance and characterised by very high loadings of pH and total alkalinity. Third factor with 8% of variance has high loadings of sulphate and nitrate which indicates the discrete sources for these ions.
Geoelectrical surveying
The thickness of different layers and apparent resistivity values obtained from 7 VES stations along with curve types are given in Table 10. The shape of the VES curve is controlled by the nature and distribution of underlying formation i.e. the resistivity and thickness of layers. Out of total seven locations, five locations depict four-layered structure in which three points show KH type, and two show QH type. Two of the remaining soundings of Kalanad river basin curves are three layered and gives Q and H type. The occurrence KH type curve (43%) reveals the presence of highly resistive top lateritic soil followed by saturated zone and the basement topography in the entire basin. Additional indication of a top resistive layer is from wide spread distribution of different combinations of H types. Therefore, it is anticipated here that the water-saturated zone is overlained by a top hard layer and underlained by another resistive layer possibly the basement. Among the three-layered curves, Q type gives low resistivity which suggests a high groundwater potential or the coastal areas where saline water prevail. Generally, ‘H’ type curves are attained in hard rock terrain consisting of dry top soil of high resistivity followed by either a water saturated or weathered layer of low resistivity and then a compact hard rock of very high resistivity. According to the present study, Q type is seen in the coastal area and the H type in the hard rock terrain.
Resistivity and thickness of layers
Spatial variation maps showing thickness and resistivity of geoelectrical layers were prepared. Resistivity of first layer (Fig. 4) ranges from 3.957 ohmm (VES location V4) to 798 ohmm (VES location V7). The spatial variation of first layer resistivity suggests high resistivity value in the eastern side of the basin, whereas resistivity value decreases towards the coastal side. Thickness of first layer is varying from 1.9 m in VES location V1 to 12.6 m in VES location V7 (Fig. 5). The maximum thickness of first layer is encountered in the northeastern part of the study area. Resistivity of second layer (Fig. 6) varies from 3.96 ohmm (VES location V2) to 1355 ohmm(VES location V7), and the second layer thickness (Fig. 7) ranges from 3.69 m (VES location V1) to 35.4 m (VES location V7).The maximum thickness of second layer is found in the coastal areas. The areas showing relatively higher values for second layer resistivity and thickness suggest the presence of a thick unsaturated layer below the surface which may be laterite. The resistivity of third layer (Fig. 8) is ranging from 0.296 ohmm (VES location V1) to 842 ohmm (VES location V7). In spatial variation map, the northeastern side shows high resistivity value for third layer which indicates the crystalline bedrocks in that area.
Depth wise isoresistivity
As reference to 5 m, l0 m, 15 m, 25 m, 30 m, 40 m, 60 m, 70 m, 80 m and 90 m depths isoresistivity maps have been prepared, and it gives an idea about the underground structure and vertical changes in resistivity. In the central and southwest portions of the basin, isoresistivity at 10 m,15 m and 25 m depth shows low resistivity zones, hence these areas are good sites for constructing open wells (Figs. 9, 10 and 11). Isoresistivity map at 70 m (Fig. 12) indicates the presence of a relatively high resistivity area which may be due to highly consolidated and compacted sedimentary beds.
Conclusions
The outcome of the present study in the Kalanad basin is given below. Comparisons of various physicochemical parameters of the water samples with BIS and WHO standards show that almost all parameters fall within the maximum permissible limit. Also, it has been observed that most regions in the study area have low pH. The low pH of the well samples can be attributed to the laterite in the study area as it has the capacity to generate acidity and utilize most of the dissolved oxygen in the water that infiltrates into the subsurface. The acidic and corrosive nature of low pH (< 6.5) water results in leaching of metal ions such as iron, manganese, copper, lead, and zinc from the aquifer and pipings. It can also cause many health problems such as stomach pain, ulcers, and hyper acidity. All water samples satisfy the various permissible standard values as per the irrigation water quality indices. The Percent sodium index parameter shows most of the water samples are found within the permissible range for irrigation purpose. All the water samples are found excellent for irrigation purposes as per the Sodium Adsorption Ratio and Residual Sodium Carbonate. The hydrogeochemical facies plot with piper trilinear diagram reveals that in majority of the samples, alkalies exceed alkaline earths, and also most of the samples belong to sodium chloride type which indicate the chances for saline water intrusion in coming years in the coastal area. Correlation analysis and third factor of R mode factor analysis indicated that sulphate and nitrate have different sources when compared with other ions. R mode factor analysis extracted three major components which constitute for 93% of total variance. Out of which, factor 1 having 72% of the variance represents TDS and permanent hardness of the water. The second factor having 12% of variance denotes pH and total alkalinity content.
Resistivity data from seven locations are interpreted qualitatively and quantitatively. It revealed that five locations have four-layered resistivity profile in which three points show KH type and two points show QH type. The remaining two soundings in Kalanad river basin curves are three layered which are Q and H type. The significant occurrence of typical KH type curve shows the presence of highly resistive top lateritic soil followed by saturated zone and then the basement in the entire basin. The common occurrence of different combinations of H types is a clear indication of a top resistive layer. The areas having relatively higher resistivity for second layer and high value for thickness suggest the presence of a thick unsaturated layer below the surface which can be laterite. In the northeastern side, there is high resistivity value for the third layer which indicates fractureless crystalline bedrocks in that area. The isoresistivity at 10 m, 15 m and 25 m depth indicates low resistivity zones in the central and southwest portions of the basin which can be good sites for constructing open wells. Isoresistivity map at 70 m indicates the occurrence of a relatively high resistivity material near to the central portions of the study area which may be the highly consolidated and compacted sedimentary beds.
References
Anon (1946) The salt problem in irrigation agriculture. United States Department of Agriculture Miscellaneous Publication, Washington, vol 607, p 27
APHA (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, Washington, p 1220
Back VV, Hanshaw BB (1965) Chemical Geohydrology. Adv. Hydrosci 2:49–109
Balakrishna S (1980) Hydro geophysical surveys in India. Presidential Address at the Indian Association of Geohydrologists, NGRI Hyderabad
Balwant K, Umesh KS, Indrani M, Basu MD (2016) Water quality status of Indian major rivers with reference to agriculture and drinking purposes. In: Raman VAV (ed) Geoanthropogenic environment an appraisal. A.K.Publications, Delhi, pp 65–81
Basak P (1992) Kerala’s water resources myths, facts and realities. Centre for Water Resources Development and Management, Kozhikode
Beeson S, Jones CRC (1988) The combined EMNES geophysical method for sitting boreholes. Ground Water 26(1):54–63
Bhattacharya PK, Patra HP (1968) Direct current geoelectric sounding, principles and interpretation, methods in geochemistry and geophysics. Elsevier, Amsterdam
BIS (2012) Specifications for drinking water, IS: 10500, 2012. New Delhi, Bureau of Indian Standards, p 18
Brindha K, NeenaVaman KV, Srinivasan K, Sathis Babu M, Elango L (2014) Identification of surface water- groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, Southern India. Appl Water Sci 4:159–174
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Non point of surface waters with phosphorous and nitrogen. EcolAppl 8(3):559–568
Chandra, PC, Athavale, RN (1979) Close grid resistivity survey for demarcating the aquifer encountered in borewell at Koyyur in Lower Maner Basin. Tech. Rpt. No. GH, p16
Chandrasekharan H (1988) Geo-electrical investigations for groundwater in Thar desert, western Rajasthan: some case studies. Transactions of Indian Society of Desert Technology and University Centre of Desert Studies. pp 155–168
Collins R, Jenkins A (1996) The impact of agricultural land use on stream chemistry in the Middle Hills of the Himalayas. Nepal J Hydrol 185(1–4):71–86
Davis JC (1973) Statistics and Data analysis in Geology. Wiiley, New York, p 550
Destadelhofen CM (1994) Anwendung Geophysikalisher Methoden in der Hydrogeologie. Springer, Berlin
Eaton EM (1950) Significance of carbonate in irrigation water. Soil Sci 69:123–133
Edmunds WM (1994) Groundwater chemistry and development, Groundwater quality. In: Nash H, McCall GJH (eds) Chapman and Hall, London
Griffith DH, King RF (1965) Applied geophysics for engineers and Geologists. Pergamon, London
Haritash AK, Gaur S, Garg S (2014) Assessment of water quality and suitability analysis of River Ganga in Rishikesh. India Appl Water Sci 6(4):383–392
Hill RA (1940) Geochemical patterns in the Coachella valley, California. Trans Am Geophys Union 21:46–49
Hill RA (1942) Salts in irrigation water. T Am Soc CivEng 107:1478–1518
Iqbal H, Inam A, Bakhtiyar Y, Inam A (2012) Effluent quality parameters for safe use in agriculture. Water Qual Soil Manag Irrig Crops 28:23–36
Jarvie HP, Whitton BA, Neal C (1998) Nitrogen and phosphorus in east coast British Rivers: speciation, sources and biological significance. Sci Total Environ 210(211):79–109
Joliffe IT (2002) Principle Component Analysis, 2nd edn. Springer, Berlin, p 488
Kaiser HF (1960) The application of electronic computers to factor analysis. Edu Psychol Measure 20:141–151
Kelley WP (1951) Alkali soils-their formation properties and reclamation. Reinhold Publication Corporation, New York
Koefoed O (1979) Geosounding Principles, (1) Resistivity sounding measurements. Elsevier, Amsterdam, p 276
Kumar SK, Rammohan V, Sahayam JD, Jeevananadam M (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha river basin, Tamil Nadu, India. Environ Monit Assess 159:341–351
Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to ground water: an introduction. Clarendon Press, Oxford, p 296
Parasins DS (1966) Principles of applied geophysics. Chapman Hall, New York
Piper AM (1944) A graphical procedure in the chemical interpretation of Groundwater analysis. Trans Am Geophys Union 25:914–923
Prasanna MV, Chidambaram S, Gireesh TV, Ali TVJ (2011) A study on hydrochemical characteristics of surface and sub-surface water in and around Perumal Lake, Cuddalore district, Tamil Nadu. South India Environ Earth Sci 63(1):31–47
Ramakrishna YS (1998) Groundwater handbook, India, p 556
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, vol 60. Agricultural Handbook, Washington, DC, p 160
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofoniou M, Kouimtzis TH (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Singh AK, Raj B, Tiwari AK, Mahato MK (2013) Evaluation of hydrogeochemical processes and groundwater quality in the Jhansi district of Bundelkhand region. India Environ Earth Sci 70(3):1225–1247
Sreedevi, (2004) Groundwater Quality of Pageru River Basin, Cuddapah District. Andhra Pradesh J Geol Soc India 64(5):619–636
Sridharan M, Senthil Nathan D (2017) Groundwater quality assessment for domestic and agriculture purposes in Puducherry region. Appl Water Sci 7(7):4037–4053
Srinivasamoorthy K, Vijayaraghavan K, Vasanthavigar M, Sarma S, Chidambaram S, Anandhan P, Manivannan R (2010) Assessment of groundwater quality with special emphasis on fluoride contamination in crystalline bed rock aquifers of Mettur region, Tamilnadu. India Arab J Geosci 5(1):83–94
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Trivedi RK, Goel PK (1986) Chemical and biological methods for water pollution studies. Environmental Publications, India, p 215
Vijayakumar N et al (2015) Land use and land cover change detection in Thirumanimuttar Sub Basin, Cauvery River, Tamilnadu. Int J Sci Eng Technol Res 4:680–683
Walton WC (1970) Groundwater resource evaluation. McGraw-Hill, New York
Ward SH (1990) Resistivity and induced polarisation methods. In geotechnical and environment geophysics, ed. Ward, S.H., Soc. of Exploration Geophysists. Tulsa, Oklahoma 1:147–190
WHO (2004) Guidelines for drinking water quality, Recommendations, vol 1. World Health Organization, Geneva
Wilcox LV (1948) The quality of water for irrigation use Technical bulletin 962. US Department of Agriculture, Washington, DC
Wilcox LV (1955) Classification and use of irrigation waters. US Department of Agriculture Circular 969, Washington, DC, pp 19–20
Zohdy AAR, Eaton GP, Mabey DR (1974) Application of surface Geophysics to groundwater investigations. USGS 1:116
Funding
Not Applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anoop, S., Ashwathi, C., Baburaj, V.H. et al. Hydrogeochemical status and geoelectrical characteristics of the shallow aquifers of Kalanad Basin, Kasaragod, Kerala, India. Appl Water Sci 11, 20 (2021). https://doi.org/10.1007/s13201-021-01361-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01361-0