Introduction
Barnacles (subclass Cirripedia) are sessile crustaceans that are commonly found attached to natural surfaces such as rocks, shells and corals (Chan & Høeg, Reference Chan, Høeg, Thiel and Watling2015). However, those in the superfamily Coronuloidea specialize as obligate commensals of mobile marine animals including mammals, reptiles, chelicerates and large crustaceans (Badrudeen, Reference Badrudeen2000; Cheang et al., Reference Cheang, Tsang, Chu, Cheng and Chan2013; Hayashi, Reference Hayashi2013; Hayashi et al., Reference Hayashi, Chan, Simon-Blecher, Watanabe, Guy-Haim, Yonezawa, Levy, Shuto and Achituv2013; Zardus et al., Reference Zardus, Lake, Frick and Rawson2014; Carrillo et al., Reference Carrillo, Overstreet, Raga and Aznar2015; Buckeridge et al., Reference Buckeridge, Chan and Lee2018, Reference Buckeridge, Chan and Lin2019; Dreyer et al., Reference Dreyer, Zardus, Høeg, Olesen, Yu and Chan2020; Kim et al., Reference Kim, Chan, Kang, Kim and Kim2020). They live as epibionts on the exterior body of their hosts of which sea turtles carry the widest variety (Frick & Pfaller, Reference Frick, Pfaller, Wyneken, Lohmann and Musick2013; Hayashi, Reference Hayashi2013); therefore, in analyses of associations between sea turtles and epibionts, much attention has been given to barnacles in particular (Zardus & Balazs, Reference Zardus and Balazs2007; Pfaller et al., Reference Pfaller, Frick, Reich, Williams and Bjorndal2008; Frick et al., Reference Frick, Zardus and Lazo-Wasem2010; Fuller et al., Reference Fuller, Broderick, Enever, Thorne and Godley2010). More than 20 nominal species of barnacles from Balanomorpha (acorn barnacles) and Lepadomorpha (gooseneck barnacles) have been reported from sea turtles (Hayashi, Reference Hayashi2009). Chelonibia testudinaria is the most cosmopolitan and largest acorn barnacle on sea turtles, reaching a diameter of 120 mm (Zardus & Hadfield, Reference Zardus and Hadfield2004), and associate with all seven species of sea turtles in the world's oceans including the flatback turtle (Natator depressus) (Monroe & Limpus, Reference Monroe and Limpus1979); as well as the dermis-covered leatherback turtle (Dermochelys coriacea) (Rees & Walker, Reference Rees and Walker1993). Of the four species of barnacles in the family Chelonibiidae, three have been found to be genetically identical and are synonymized under C. testudinaria (Zardus et al., Reference Zardus, Lake, Frick and Rawson2014). For individuals attached to sea turtles, a diagnostic trait of C. testudinaria (Figure 1) is its stellate shell patterning formed by open wedges at the sutures between the shell plates or compartments, sculpted along their margins with indentations (Darwin, Reference Darwin1854; Hayashi, Reference Hayashi2013).

Fig. 1. A large, single hermaphroditic individual of the turtle barnacle Chelonibia testudinaria from a green turtle, demonstrating how size was measured (maximal rostro-carinal diameter) and the positioning of small complemental males attached to its shell (scale bar = 10 mm). Photo by Kah Kheng Lim.
Parasitic epibionts of sea turtles, leeches for instance, which derive nutrition from the tissues of their hosts can significantly impact the health of sea turtles (Greenblatt et al., Reference Greenblatt, Work, Balazs, Sutton, Casey and Casey2004). In contrast, barnacles and other commensals use sea turtles primarily as a substratum or foraging platform (Frick et al., Reference Frick, Williams and Veljacic2002) with minor or equivocal impacts on the turtles' health (Stamper et al., Reference Stamper, Harms, Epperly, Braun-McNeill and Stoskopf2005; Flint et al., Reference Flint, Morton, Limpus, Patterson-Kane, Murray and Mills2010). Depending on the species of barnacle, they fasten onto the carapace and plastron, on the head, or the flippers and skin of their host turtles (Frick & Ross, Reference Frick and Ross2001; Devin & Sadeghi, Reference Devin and Sadeghi2010; Ooi & Palaniappan, Reference Ooi and Palaniappan2011; Nájera-Hillman et al., Reference Nájera-Hillman, Bass and Buckham2012). There are two types of attachment modes across different life stages of barnacles. First, the cypris larva arrives on the substratum and secretes cyprid cement from multicellular cement glands that lead to ducts on the antennules. The release of this cement triggers the metamorphosis of the cypris larva into juvenile and adult barnacles. Second, the adult secretes cement from cement glands to maintain firm attachment. Both stages secrete cement from cement glands that lead through the base (Lacombe, Reference Lacombe1970; Walker, Reference Walker1978).
How the sea turtle life cycle influences barnacle occurrence is unresolved. Most sea turtles undergo long-distance migrations to different habitats throughout their lives (Åkesson et al., Reference Åkesson, Broderick, Glen, Godley, Luschi, Papi and Hays2003). At hatching, newly emerged sea turtles migrate toward oceanic nursery areas then, as older juveniles, subadults and adults, return to neritic foraging grounds (Musick & Limpus, Reference Musick, Limpus, Lutz and Musick1997; Lohmann et al., Reference Lohmann, Putman and Lohmann2008). A recent study by Burgett et al. (Reference Burgett, Burkholder, Coastes, Fourqurean, Kenworthy, Manuel, Outerbridge and Fourqurean2018) has confirmed the ontogenetic diet shifts of green turtles (Chelonia mydas) in a mid-ocean developmental habitat, thus supporting the flexibility of habitat shifts between their foraging and neritic habitats (Hayashi & Nishizawa, Reference Hayashi and Nishizawa2015). Mature adults periodically migrate between distantly spaced breeding grounds, nesting beaches and foraging areas with a high degree of fidelity for these areas (Bjorndal, Reference Bjorndal1995; Palaniappan & Abd Hamid, Reference Palaniappan and Abd Hamid2017; Shimada et al., Reference Shimada, Limpus, Hamann, Bell, Esteban, Groom and Hays2020). A foraging turtle is defined as a sea turtle that resides in an area where its food source is available (Ceriani et al., Reference Ceriani, Roth, Evans, Weishampel and Ehrhart2012; Cheng et al., Reference Cheng, Chan, Hong, Johnson and Cheng2019) and exhibits a strong long-term fidelity to localized foraging sites whereas a resident turtle is defined as a non-migratory individual (Márquez, Reference Márquez1990), that after recruiting from its oceanic nursery resides predominantly at the foraging ground and thus has the probability of being recaptured throughout its resident years (Chaloupka & Limpus, Reference Chaloupka and Limpus2001). Foraging turtle aggregations commonly comprise several nesting stocks (Nishizawa et al., Reference Nishizawa, Joseph and Chong2016). Both foraging and resident female turtles will not nest in the foraging areas. Instead, they return to their natal beach to nest due to their natal homing nature. Nesting turtles are reproductively active females that migrate from distant, long-term residence areas (hereafter, foraging grounds) to their natal nesting beaches during the breeding season (Ceriani et al., Reference Ceriani, Weishampel, Ehrhart, Mansfield and Wunder2017; Sönmez, Reference Sönmez2019). Documenting the epibiont diversity associated with migrating sea turtles can help provide information about where epibiosis occurs and hence the migratory behaviour and habitat preferences of sea turtles (Hayashi, Reference Hayashi2009). The findings of Robinson et al. (Reference Robinson, Lazo-Wasem, Paladino, Zardus and Pinou2017) suggest that sea turtle epibiont communities are more reflective of where sea turtles feed than where they nest. In addition, stable isotope analysis of barnacle shells has been used to provide insight on foraging distributions, migration distances and habitat use of nesting turtles over time (Pearson et al., Reference Pearson, van de Merwe, Gagan, Limpus and Connolly2019), thus aiding in sea turtle population conservation and management.
An initial study of sea turtle epibionts for the region of Sabah, Malaysia by Ooi & Palaniappan (Reference Ooi and Palaniappan2011) found only one species of barnacle on sea turtles – C. testudinaria, occurring on both green Chelonia mydas (Linnaeus, 1758) and hawksbill Eretmochelys imbricata (Linnaeus, 1766) turtles in the waters of Mabul and Sipadan Islands. Mabul Island (Figure 2) is home to a variety of seagrass species especially Thalassia hemprichii and diverse coral reef communities (Jolis & Kassem, Reference Jolis and Kassem2011), which attracts a foraging sea turtle population dominated by green turtles, and a small number of hawksbill turtles. So far as is known, both sea turtle species are residents in Mabul Island and some have been caught repeatedly over consecutive years of sampling. Hawksbill and green turtles also share foraging grounds at neighbouring Sipadan Island (14.5 km away) (Chong, Reference Chong2012; Joseph et al., Reference Joseph, Nishizawa, Hassan, Zakariah, Jaaman and Zhang2017). Only green turtles hatched in Mabul Island will travel back to their natal beach to nest during the breeding season then return to their foraging grounds, as reported by Joseph et al. (Reference Joseph, Nishizawa, Hassan, Zakariah, Jaaman and Zhang2017).

Fig. 2. Map of Mabul Island, south-eastern Sabah, Malaysia, showing established dive sites where sea turtles were captured. Map by Haziq Harith Abd Hamid.
In the course of examining the sea turtles in Mabul Island, many were found hosting the barnacle C. testudinaria (Figure 1). In addition, many of the barnacles were found with multiple small male individuals attached to larger hermaphrodites, situated within the narrowest portions of the hermaphrodites' settlement pits as described by Zardus & Hadfield (Reference Zardus and Hadfield2004) or attached randomly on the shell surface and apertural orifice (Cheang et al., Reference Cheang, Tsang, Chu, Cheng and Chan2013). Barnacles in general are hermaphroditic and cross-fertilize with neighbouring individuals via the typical barnacle mode of pseudo-copulation (they deposit sperm into a partner's mantle cavity) (Murata et al., Reference Murata, Imafuku and Abe2001), though some barnacle species are capable of performing sperm casting with subsequent fertilization of the internally kept broods (Barazandeh et al., Reference Barazandeh, Davis, Neufeld, Coltman and Palmer2013). Darwin (Reference Darwin1854) was the first to describe the rare phenomenon of androdioecious reproduction in scalpellid barnacles, wherein small males fertilize larger hermaphrodites, naming the small companions as complemental males. Zardus & Hadfield (Reference Zardus and Hadfield2004) found that the complemental males of C. testudinaria possess mature sperm and serve as sperm donors, however it is unknown if complemental males are protandrous and in time develop into full-grown hermaphrodites (Weeks et al., Reference Weeks, Benvenuto and Reed2006) or whether they can flexibly develop female sexual characters according to their position and partner availability (Wijayanti & Yusa, Reference Wijayanti and Yusa2016).
Most sea turtle barnacle investigations have focused on barnacle abundance and diversity relative to sea turtle species (Hernández–Vázquez & Valadez–González, Reference Hernández-Vázquez and Valadez-González1998; Pfaller et al., Reference Pfaller, Bjorndal, Reich, Williams and Frick2006, Reference Pfaller, Frick, Reich, Williams and Bjorndal2008; Fuller et al., Reference Fuller, Broderick, Enever, Thorne and Godley2010). This study, in addition to barnacle occurrence, presents fundamental information on the occurrence of complemental males of C. testudinaria as well. To date, few studies have included information on the complemental males of C. testudinaria from wild sea turtles. Ewers-Saucedo et al. (Reference Ewers-Saucedo, Arendt, Wares and Rittschof2015) studying C. testudinaria in the south-eastern USA, determined that complemental males were more commonly partners with hermaphrodites from loggerhead sea turtles (Caretta caretta) than with hermaphrodites from crabs, perhaps due to smaller group size and higher mortality rates associated with the latter hosts. The objective of the present study was to determine whether the abundance and size class distribution of the epibiotic barnacle, C. testudinaria, and the occurrence of its complemental males, in the foraging population of sea turtles at Mabul Island, is influenced by sea turtle species and life stage.
Materials and methods
The study was conducted on the foraging population of sea turtles at Mabul Island (4.25°N 118.63°E) located on the east coast of Sabah, Malaysia (Figure 2). Sea turtles were caught in-water from May 2015 to November 2017. Fieldwork was conducted over a period of 4–6 days once every 6 months per year (a total of 26 days spread over 6 sampling trips) to keep disturbance to the sea turtles at a minimum. The sea turtles were randomly caught by hand while scuba diving during the day at established dive sites (distance between adjacent dive sites did not exceed 500 m) in Mabul Island at depths not exceeding 20 m. The sea turtles were divided into three life stages based on size i.e. straight carapace length (SCL), namely juvenile, sub-adult and adult, with adults further divided into female and male following previous studies (Bresette et al., Reference Bresette, Witherington, Herren, Bagley, Gorham, Traxler, Crady and Hardy2010; Palaniappan, Reference Palaniappan2017). Sex distinctions were based on size of the tail (van Dam & Diez, Reference van Dam and Diez1998). The sea turtles were brought onto the research vessel to be photographed, measured and tagged. The SCL of each sea turtle was measured with 1.0 m Mitutoyo stainless Vernier callipers (accurate to 0.05 ± 0.15 mm) measuring from the anterior point at the midline (nuchal scute) to the posterior end of the supracaudal (Bolten, Reference Bolten, Ecker, Bjorndal, Abreu-Gobrois and Donnelly1999). Inconel tags (National Band and Tag Company, Newport, Kentucky, USA) were applied to the sea turtles at the posterior edge of both front flippers.
Scaled photographs were taken of the specific locations of the barnacles found on the body of the sea turtles. For each turtle, including repeat captures, all attached barnacles larger than 5 mm were removed before release. Barnacles found on the head, carapace and plastron of the sea turtles were gently removed using a hammer and scraper. Barnacles found elsewhere on the sea turtle were not included in this study. The collected barnacle specimens (minimum size 5 mm) were kept in resealable plastic bags labelled with the turtle's ID number, date of collection and the location that they were found on the turtle's body. The barnacles were identified in the laboratory at the Borneo Marine Research Institute, Universiti Malaysia Sabah (UMS) to the lowest taxonomic level following Monroe (Reference Monroe1981). Barnacle size was measured as maximal rostro-carinal diameter (Figure 1) with 0.3 m Mitutoyo stainless steel Vernier callipers (accurate to 0.02 ± 0.04 mm). The complemental males that were found attached on every hermaphrodite were examined and counted under a Carl Zeiss Stemi stereomicroscope. Barnacles with broken shells were excluded from the analysis.
Results and discussion
Field survey results
A total of 403 sea turtles were captured from May 2015 to November 2017 in Mabul Island, consisting of 364 green turtles and 39 hawksbill turtles. All life stage groups, as determined by SCL, were represented in the population of green turtles (77.8% juveniles, 6.0% sub-adults, 6.9% adult males, 9.3% adult females) but hawksbill turtles were mostly juveniles (94.9%) with only one sub-adult and one adult female (Table 1). The low number of sub-adult and adult hawksbill turtles suggests that juvenile hawksbill turtles eventually move to neritic habitats and utilize Mabul Island as a temporary developmental foraging ground (Pilcher, Reference Pilcher2010; Joseph et al., Reference Joseph, Nishizawa, Hassan, Zakariah, Jaaman and Zhang2017).
Table 1. Number of sea turtles (N) with and without barnacles in Mabul Island, by turtle species and life stage. Measurements shown are the straight carapace lengths (SCLs) of the turtles

SD, standard deviation.
Mabul Island consists of a mixed population of foraging, resident and nesting sea turtles. All the sea turtles caught in-water during this study were tagged upon capture. We have had no reports of these tag numbers among the green turtles that have nested in Mabul Island. Green turtles show natal homing on a very broad, regional scale well before their first reproductive migration (Allard et al., Reference Allard, Miyamoto, Bjorndal, Bolten and Bowen1994). When sea turtles leave the open ocean to establish coastal feeding sites, they choose foraging grounds within their general natal region, which are often a considerable distance from the natal beach (Bowen et al., Reference Bowen, Bass, Chow, Bostrom, Bjorndal, Bolten, Okuyama, Bolker, Epperly, Lacasella, Shaver, Dodd, Hopkins-Murphy, Musick, Swingle, Rankin-Baransky, Teas, Witzell and Dutton2004; Bowen & Karl, Reference Bowen and Karl2007). A total of 2255 barnacles summed across all hosts were collected, all of a single species, Chelonibia testudinaria. This is consistent with previous studies of green turtles in Mabul and Sipadan Islands, Malaysia (Ooi & Palaniappan, Reference Ooi and Palaniappan2011) and in the waters of Japan (Hayashi & Tsuji, Reference Hayashi and Tsuji2008). Intriguingly, Dobbs & Landry (Reference Dobbs and Landry2004) did not find this barnacle species in a nesting population of Australian hawksbill turtles but did document the presence of other species of commensal coronulids, including Chelonibia caretta (the dominant species with 81% occurrence), Platylepas sp. and the burrowing barnacle Chelolepas cheloniae. Nevertheless, a recent study by Razaghian et al. (Reference Razaghian, Esfandabad, Hesni, Shoushtari, Toranjzar and Miller2019) had reported C. testudinaria on the nesting hawksbill turtles in Iran. The overall incidence of sea turtles carrying this barnacle was similar between the two host species with 208 green turtles (57.1%) and 22 hawksbill turtles (56.4%) hosting it. The percentage of sea turtles with C. testudinaria was also similar (~50%) across the host life stages present (Table 1) with no significant difference (χ2(3) = 0.15, P > 0.05). However, the quantity of barnacles per turtle varied with turtle size, species and sex.
Abundance and size variation of C. testudinaria
Overall, on sea turtles with barnacles, barnacle abundance ranged from 1–232 individuals per turtle (mean = 9.80 individuals ± 23.20 SD), across both hosts. The highest number of barnacles was found on a large adult female green turtle (SCL = 910 mm) whereas several individuals of both host species hosted only one barnacle each. In comparison between the two sea turtle species, juvenile green turtles had substantially more barnacles (mean = 6.9 individuals ± 9.34 SD) than juvenile hawksbill turtles (mean = 3.5 individuals ± 2.98 SD) (Figure 3). This could be due to the greater body surface area of green turtles which on average is larger than hawksbill turtles, a finding that is consistent with Hayashi & Tsuji (Reference Hayashi and Tsuji2008). There was a statistically significant difference between the sea turtle life stages on the combined dependent variables (i.e. barnacle abundance and size of barnacles) after controlling for turtle size (SCL) and turtle species (F(8, 428) = 5.77, P < 0.001, Pillai = 0.19). We found, to a lesser degree, C. testudinaria on the foraging population of hawksbill turtles in Mabul Island. The small sample size of adult hawksbill turtles captured in this study did not allow us to fully address the relationship between the abundance of barnacles and turtle size, therefore, no further analyses were performed.

Fig. 3. Frequency distribution of Mabul Island green and hawksbill turtles, with and without barnacles, grouped by turtle straight carapace length (SCL) and life stage: juvenile (J), sub-adult (SA), adult male (AM) and adult female (AF).
Among green turtles, females had considerably more barnacles (mean = 43.4 individuals ± 67.96 SD) than males (mean = 12.4 individuals ± 14.39 SD), sub-adults (mean = 14.5 individuals ± 14.79 SD) and juveniles (mean = 6.9 individuals ± 9.34 SD). Few studies have compared barnacle occurrence among sea turtles in populations of mixed life stages though several have compared epibionts among mixed sea turtle species (Fuller et al., Reference Fuller, Broderick, Enever, Thorne and Godley2010; Lazo-Wasem et al., Reference Lazo-Wasem, Pinou, Peña de Niz and Feuerstein2011; Ooi & Palaniappan, Reference Ooi and Palaniappan2011; Robinson et al., Reference Robinson, Lazo-Wasem, Paladino, Zardus and Pinou2017). The number of barnacles per turtle varied widely in our study with the highest number (i.e. 232 individuals) collected from an adult female at Mabul Island, considered a high amount for green turtles (Bugoni et al., Reference Bugoni, Krause, de Almeida and de Pádua Bueno2001; Hayashi & Tsuji, Reference Hayashi and Tsuji2008; Fuller et al., Reference Fuller, Broderick, Enever, Thorne and Godley2010; Lazo-Wasem et al., Reference Lazo-Wasem, Pinou, Peña de Niz and Feuerstein2011; Nájera-Hillman et al., Reference Nájera-Hillman, Bass and Buckham2012; Robinson et al., Reference Robinson, Lazo-Wasem, Paladino, Zardus and Pinou2017). Our results indicate that the number of barnacles attached to green turtles increases with turtle size, and this finding is consistent with Hayashi & Tsuji (Reference Hayashi and Tsuji2008) and Devin & Sadeghi (Reference Devin and Sadeghi2010). The attachment area selected is due to the free space availability (Minchinton & Scheibling, Reference Minchinton and Scheibling1993; Ihwan et al., Reference Ihwan, Joseph, Jamaan, Wahidah and Marina2018), thus resulting in a higher abundance of barnacles in larger sea turtles.
The size of barnacles differed significantly between collecting trips as determined by one-way ANOVA (F(5, 2249) = 15.18, P < 0.001), except those collected in May of 2015 and 2016. Barnacles collected in May were larger (mean = 19.1 mm ± 8.41 SD) than those collected in November (mean = 16.7 mm ± 8.81 SD), across all sampling trips, indicative of either periodic acquisition or growth. The size frequency distribution of barnacles across the duration of the study was skewed significantly from normal as determined by the Shapiro–Wilk's test (Shapiro & Wilk, Reference Shapiro and Wilk1965) (W = 0.95363, P < 0.001). A skewness test confirmed the data were skewed to the right, driven by the presence of a few large individuals (Figure 4). However, Hartigan's dip test (Hartigan & Hartigan, Reference Hartigan and Hartigan1985) for unimodality indicated our data had a single peak (i.e. lacked discrete size classes), at 5.1–10 mm (Figure 4). Even though we found that barnacles in Mabul Island collected in May were generally larger than those collected in November, the size frequency distribution of the barnacles was unimodal. This result is contradictory to Ewers-Saucedo et al. (Reference Ewers-Saucedo, Arendt, Wares and Rittschof2015) and Ten et al. (Reference Ten, Pascual, Pérez-Gabaldón, Tomás, Domènech and Aznar2019) where they detected two different age classes of C. testudinaria in their studies. Age estimation of barnacles as a function of their size has been examined in past studies (Ewers-Saucedo et al., Reference Ewers-Saucedo, Arendt, Wares and Rittschof2015; Doell et al., Reference Doell, Connolly, Limpus, Pearson and van de Merwe2017; Ten et al., Reference Ten, Pascual, Pérez-Gabaldón, Tomás, Domènech and Aznar2019). Ewers-Saucedo et al. (Reference Ewers-Saucedo, Arendt, Wares and Rittschof2015) suggested that the size frequency distribution of each age class in a natural population should be normally distributed and multimodal if several age classes are present. While our data were positively skewed, there was no evidence of multimodality in our data despite the presence of a few large individuals on host sea turtles. A lack of age classes in our study may be due to year-round reproduction of C. testudinaria in a tropical location compared with the previously mentioned studies from more seasonally influenced climates. Upon visual inspection, most of the barnacles collected in Mabul Island were in the size class of 5.1–10 mm, suggesting that these individuals were rather young. Ten et al. (Reference Ten, Pascual, Pérez-Gabaldón, Tomás, Domènech and Aznar2019) suggested that the distribution of C. testudinaria in neritic waters could result from synergistic effects of its flexibility in host selection and its short planktonic phase (about 9 days).

Fig. 4. Histogram showing the size frequency distribution of the turtle barnacle, Chelonibia testudinaria on sea turtles in Mabul Island.
Among the juvenile green turtles, large juveniles (501–750 mm) acquired larger barnacles (mean = 19.8 ± 9.11 SD) than the small juveniles (351–500 mm) (mean = 17.9 ± 7.09 SD) after controlling for SCL (F(2, 158) = 4.93, P < 0.05, Pillai = 0.06) (Figure 5A). Such difference in barnacle size between the two juvenile groups suggests these barnacle individuals are new recruits from different locations. Post-hatchling sea turtles can travel up to 12,000 km from their natal regions to juvenile foraging sites (Hays & Scott, Reference Hays and Scott2013), and might be colonized by various epibionts including barnacles along their migration pathways. The presence of bigger barnacles on larger juvenile green turtles suggests that they have acquired the barnacles prior to arrival in Mabul Island as the growth rate of the colonized barnacles is likely driven by enhanced feeding conditions caused by their host turtle's travelling activity (Trager et al., Reference Trager, Hwang and Strickler1990; Doell et al., Reference Doell, Connolly, Limpus, Pearson and van de Merwe2017).
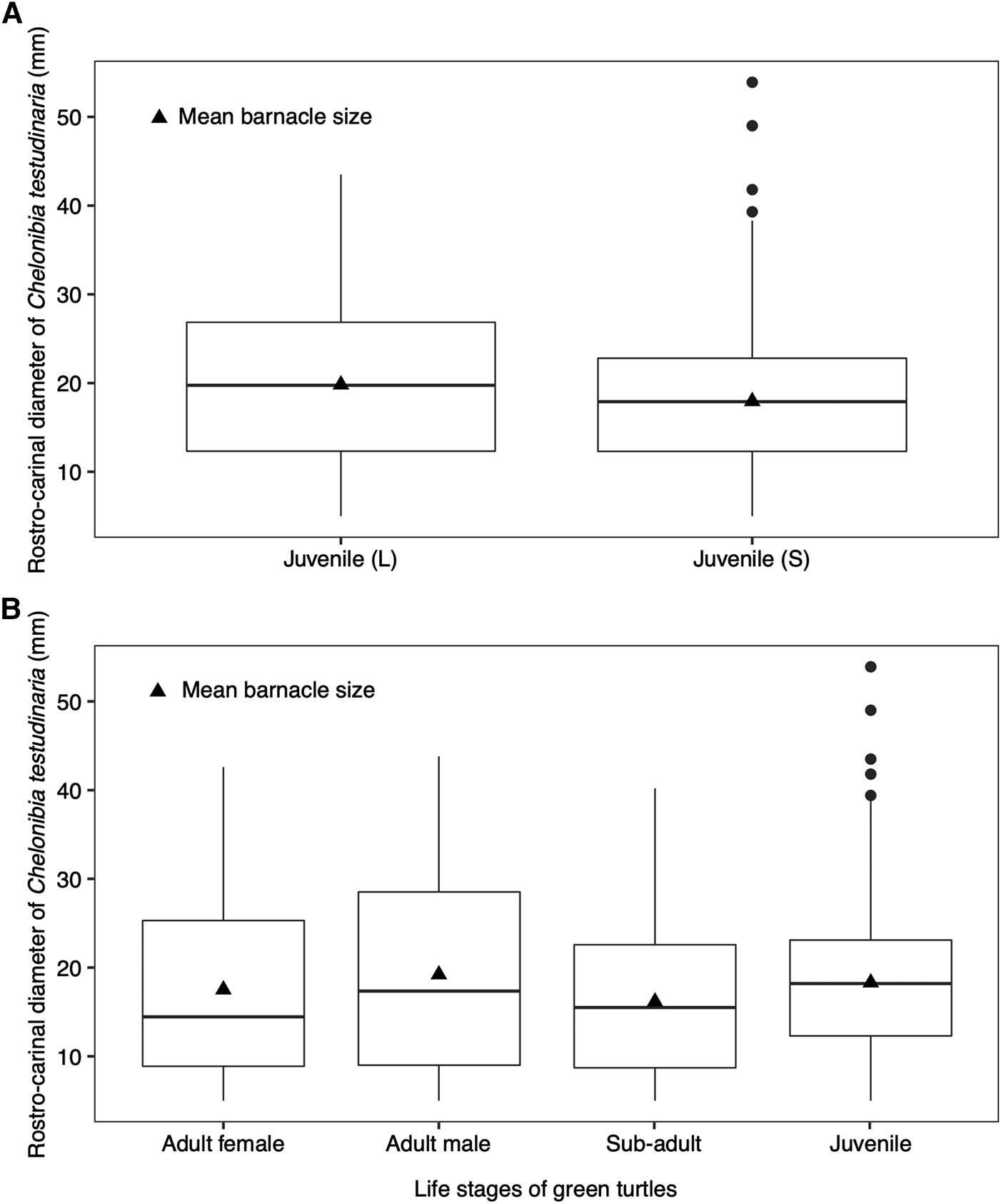
Fig. 5. Boxplots showing the distribution of rostro-carinal diameter of Chelonibia testudinaria with mean barnacle size (A) between large (L; SCL: 501–750 mm) and small (S; SCL: 351–500 mm) juvenile green turtles and (B) across the green turtle life stages. Dots represent the outliers.
We conducted an ANCOVA test on barnacle size between the foraging adult male and female green turtles, with turtle body location as the covariate. While the size of barnacles found on the plastron, carapace and head of sea turtles differed significantly (F(3, 906) = 3.58, P < 0.05), the interaction between the body location and the sex of sea turtles had no effect on the barnacle size (F(1, 905) = 0.04, P > 0.05). Nevertheless, the foraging adult males had acquired larger barnacles as compared with the foraging adult females (Figure 5B). This difference could be caused by variation in each sea turtle's historical environmental exposure (Stamper et al., Reference Stamper, Harms, Epperly, Braun-McNeill and Stoskopf2005). Male sea turtles migrate between foraging and breeding grounds more frequently (on an annual basis) compared with female sea turtles (once every 2–3 years or more) (Balazs, Reference Balazs1983; Hays et al., Reference Hays, Mazaris and Schofield2014). The growth rate of C. testudinaria is attributed to food availability, which is dependent on the sea turtle's activity level, diet and feeding behaviour (Doell et al., Reference Doell, Connolly, Limpus, Pearson and van de Merwe2017). The travelling activity associated with migrating sea turtles can cause an increase in water flow and nutrient availability, providing potentially enhanced feeding conditions for barnacles (Trager et al., Reference Trager, Hwang and Strickler1990), which may promote rapid growth of barnacles on host sea turtles (Doell et al., Reference Doell, Connolly, Limpus, Pearson and van de Merwe2017). This may explain why adult male green turtles had larger barnacles as compared with females in our study.
Barnacle site selection
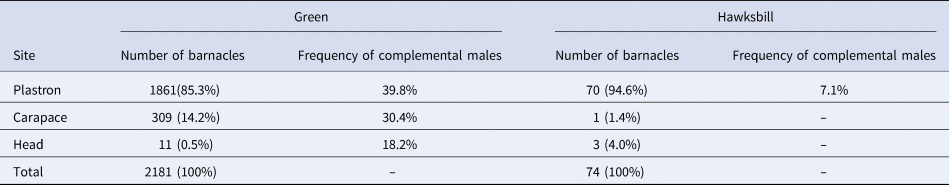
Enumeration of barnacles by body location for each sea turtle species of Mabul Island revealed a consistent pattern, that barnacles had a strong predisposition for the plastron (85.6%) over the carapace (13.7%) and low affinity for the head (0.6%) (Table 2). Among green turtles, although the foraging females had more barnacles than foraging males, the number of barnacles by body location is consistent for both sexes where higher numbers of barnacles were found on the plastron on both adult male and female turtles compared with the carapace. The occurrence of C. testudinaria on sea turtles is affected by the host sea turtle's behaviour (Frick & McFall, Reference Frick and McFall2007), size (Hayashi & Tsuji, Reference Hayashi and Tsuji2008), interactions among epibionts (Pfaller et al., Reference Pfaller, Bjorndal, Reich, Williams and Frick2006), and tolerance to desiccation and physical trauma (Pfaller et al., Reference Pfaller, Frick, Reich, Williams and Bjorndal2008). Swimming and resting behaviours displayed by sea turtles on hard substrates may affect the settlement of barnacles on a turtle's body (Razaghian et al., Reference Razaghian, Esfandabad, Hesni, Shoushtari, Toranjzar and Miller2019), while an increase in water flow could affect both settlement patterns of the larvae (Larsson & Jonsson, Reference Larsson and Jonsson2006) and further augment the feeding opportunities of the barnacles (Schärer, Reference Schärer2003). Sea turtles commonly visit specific cleaning stations on reefs to seek ‘free’ cleaning services by fishes and shrimps to get rid of inconvenient epibionts and parasites (Sazima et al., Reference Sazima, Grossman and Sazima2004; Grossman et al., Reference Grossman, Sazima, Bellini and Sazima2006; Schofield et al., Reference Schofield, Katselidis, Dimopoulos, Pantis and Hays2006; Ooi & Palaniappan, Reference Ooi and Palaniappan2011). Cleaning by most reef fish focuses on the carapace and skin of the sea turtles (Sazima et al., Reference Sazima, Grossman and Sazima2010) while the sea turtles rest on the reef motionless with the plastron facing down. Thus, small barnacles attached on the carapace may get removed more frequently than on the plastron due to higher exposure to the cleaner organisms. The presence of distinct sea turtle cleaning stations in Sipadan Island (Ooi & Palaniappan, Reference Ooi and Palaniappan2011) supports this hypothesis. Moreover, healthy sea turtles always practice self-cleaning by wedging themselves into coral crevices or scraping their carapace against ledges and other hard substrata to remove barnacles (Frick & McFall, Reference Frick and McFall2007), which may also help explain why more barnacles were found on the plastron compared with the carapace. While there was no difference between the enumeration of barnacles by body location among the adult green turtles, this suggests that both sexes share the same cleaning behaviour in Mabul Island.
Table 2. Occurrence of barnacles on selected locations of the host body along with the frequency of complemental males for green and hawksbill turtles in Mabul Island
Reacquisition of barnacles on recaptured turtles
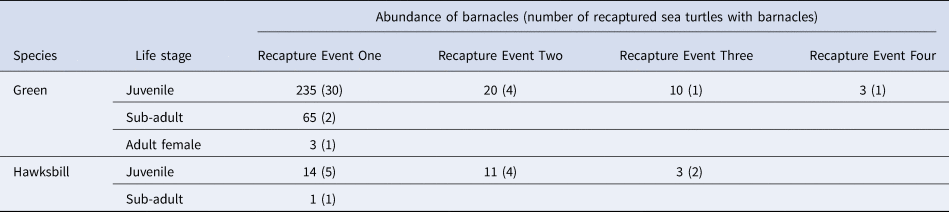
Our study is one of the few to report on reacquisition of barnacles on sea turtles. Of the 230 sea turtles with barnacles, 51 turtles were recaptured individuals (hereafter, resident sea turtles) from which all barnacles larger than 5 mm had been collected for analysis at each previous capture. Among these resident sea turtles, 39 turtles were recaptured once, eight turtles were recaptured twice, three turtles were recaptured thrice and one turtle was recaptured four times. The total quantity of reacquired barnacles was 365 (16.2% of 2255) and, among turtle life stages, was highest for juveniles for both host species (Table 3). The shortest interval for barnacle reacquisition was ~175 days (one sampling trip) whereas the longest was ~730 days (four sampling trips). The geographic localities where sea turtles are colonized by barnacles can give an understanding of the movements and habitat use by sea turtles (Ten et al., Reference Ten, Pascual, Pérez-Gabaldón, Tomás, Domènech and Aznar2019) and conversely, where and when barnacles accumulate on sea turtles can provide insight in barnacle life history. There were no large turtles (i.e. sub-adult, adult males and adult females) recaptured after Recapture Event One, but repeated captures after the first recapture were evident among juvenile turtles (Table 3). This could be attributed to the site-fidelity of juvenile turtles, similar to the findings of Pilcher (Reference Pilcher2010) where the author reported that young sea turtles exhibited minimal movement within their foraging areas over several recaptures in Mantanani Island (west coast of Sabah). The reacquisition of barnacles on recaptured juvenile turtles in our study verifies that Mabul Island is at least one source for barnacle larvae in the region.
Table 3. Abundance of reacquired barnacles for recaptured sea turtles by turtle species and life stage
Complemental males of C. testudinaria
Complemental males of the turtle barnacle C. testudinaria were encountered on 37.5% of the barnacles from the sea turtles in Mabul Island, constituting the first report of complemental males for this species in the region (Table 4). The occurrence of complemental males was greater with barnacles from green turtles (38.3%) than with barnacles from hawksbill turtles (6.8%). But, lacking larger sample sizes across other life stages of hawksbill turtles, how meaningful this difference is remains uncertain. Turtle barnacles with attached complemental males were common in this study. The complemental males of C. testudinaria have a unique settlement pattern among barnacles. They attach to the shells of hermaphrodite adults in the depressions between the shell plates, which are perhaps specialized for their settlement (Zardus & Hadfield, Reference Zardus and Hadfield2004), or at the margin of the orifice of the hermaphrodite (Crisp, Reference Crisp1983). The size of the hermaphrodites with complemental males ranged from 6.3–49.0 mm in rostro-carinal diameter (mean = 24.75 mm ± 6.86 SD) and the maximum number of complemental males arranged on a single adult hermaphrodite (25.3 mm) was 34 (mean = 3.4 individuals ± 5.93 SD). The mean size of adult hermaphrodites of C. testudinaria in Mabul Island was about 50% smaller than in other studies (Zardus et al., Reference Zardus, Lake, Frick and Rawson2014) while the maximum number of attached complemental males (N = 34) was a little more than 100% greater. The causative factor for this difference remains unknown. It is common for several complemental males to occur on a single hermaphrodite and for multiple complemental males to be found within a single settlement pit as described in Zardus & Hadfield (Reference Zardus and Hadfield2004). The males that attach nearest to the opercular rim, where the depressions are wider and are larger, presumably have the advantage of growing larger and of having a better success rate in copulating with their ‘host’ hermaphrodite (Ewers-Saucedo et al., Reference Ewers-Saucedo, Hope and Wares2016). Complemental males in the present study were mostly found attached away from the opercular rim within the ‘settlement pits’ of the adult hermaphrodite shell where they were protected from abrasion and greater risk of dislodgement (Zardus & Hadfield, Reference Zardus and Hadfield2004).
Table 4. Size of barnacle hermaphrodites with and without complemental males from selected locations on the body of host sea turtles in Mabul Island

Measurements shown are rostro-carinal diameter of the barnacles (mm) plus or minus the standard deviation (SD) and the number sampled (N).
Hermaphrodite size was on average larger for those with complemental males than for those without, but varied by their location on the body of the sea turtles (Table 4). For instance, although hermaphrodites collected from the head were generally smaller than those of the plastron and carapace, it was the larger of the head-attached individuals that hosted complemental males. Though adult hermaphroditic barnacles appear to prefer the plastron to the carapace and head of sea turtles, there was no detectable difference in the number of complemental males associated with hermaphrodites from any body location after controlling for the effect of hermaphrodites' size, (F(2, 841) = 1.99, P > 0.05). Complemental males were found on hermaphrodites from all regions of the turtle body, but less frequently on the heads of sea turtles, where the overall size of adult hermaphrodites was the smallest, suggesting that substratum space availability is the key for settlement. Complemental males in androdioecious species may evolve under lower population densities (e.g. mating group size; Yamaguchi et al., Reference Yamaguchi, Yusa, Yamato, Urano and Takahashi2008; Dreyer et al., Reference Dreyer, Sørensen, Yusa, Sawada, Nash, Svennevig and Høeg2018a) where hermaphrodite-hermaphrodite sperm competition is relaxed and chances of fertilizing the broods of a hermaphrodite partner become greater (Ewers-Saucedo et al., Reference Ewers-Saucedo, Hope and Wares2016). In these conditions androdioecy increases opportunities for fertilization more than can be realized with two separate and autonomous sexes (Kelly & Sanford, Reference Kelly and Sanford2010). Selection may also favour the small size of complemental males in C. testudinaria particularly by reducing the weight of mating units and minimizing hydrodynamic drag, thus lengthening the lives of the attached barnacles (Zardus & Hadfield, Reference Zardus and Hadfield2004). An ANOVA test verifies that the total number of complemental males increases with the size of the adult hermaphrodite (F(1, 841) = 21,74, P < 0.001). Our results suggest that large hermaphrodites host higher numbers of small complemental males, likely linked to space availability (Minchinton & Scheibling, Reference Minchinton and Scheibling1993). Hence, we hypothesize that a larger hermaphrodite would have a larger settlement pit, thus can house more cyprids that will specifically develop into males. The settlement pits of C. testudinaria can be analogized with the receptacles (areas on the rim of mantle cavity; Dreyer et al., Reference Dreyer, Yusa, Gale, Melzer, Yamato and Høeg2018b) of the androdioecious barnacle Scapellum scapellum, where the small complemental males are exclusively attached in the receptacle area of a hermaphrodite partner and never elsewhere (Spremberg et al., Reference Spremberg, Høeg, Buhl-Mortensen and Yusa2012; Høeg et al., Reference Høeg, Yusa and Dreyer2016). Dreyer et al. (Reference Dreyer, Yusa, Gale, Melzer, Yamato and Høeg2018b) found that inward curvature of the ventral transparent lamellae (a thin, cuticular membrane between the mantle rim and mantle cavity) in the receptacle area provides more space for cyprid settlement in larger species, a similar reasoning that can apply to the settlement pits of larger C. testudinaria. However, addressing this hypothesis requires additional measurements of the size of the pits on each hermaphrodite.
In conclusion, the turtle barnacle C. testudinaria was found in abundance on the foraging populations of sea turtles in Mabul Island. The highest number of barnacles found on an individual sea turtle was 232 and barnacle abundance was slightly affected by turtle size only for green turtles. Colonization of this barnacle species was the highest among adult female turtles with SCL of 828–991 mm. The majority of hawksbill turtles that were captured in the current study were juveniles, suggesting that Mabul Island serves as a temporary developmental foraging ground for this species. The repeated captures of juvenile turtles over several sampling trips, together with the abundant small-sized barnacles found among the smaller juvenile green turtles, suggest that barnacle larvae were present in Mabul Island. Among the adult green turtles, adult males had larger barnacles compared with the adult females. It is generally known that the adult males travel more frequently than females during the breeding seasons. Such intensive travelling activity may directly enhance barnacle feeding conditions, thus leading to an increase in the growth rate of barnacles on the adult male turtles particularly. Although the overall sizes of barnacles collected in May were larger than those collected in November, we were unable to relate this to seasonality due to the limited duration of sampling. The size frequency distribution of C. testudinaria in the current study was unimodal and not normally distributed. Barnacles were found to recolonize the same sea turtles several times, but the factor that drove the temporal succession of barnacles on these turtles is unclear. The highest abundance of C. testudinaria was found on the plastron (85.6%), followed by the carapace (13.7%) and the least on the head (0.6%) of sea turtles. Reacquisition of barnacles on recaptured turtles and the presence of complemental males of C. testudinaria were recorded for the first time on the foraging population of sea turtles in Mabul Island. More complemental males were found on the larger hermaphrodites, suggesting that substratum space availability is a factor in complemental male recruitment. This is supported in scalpellid barnacles, where the size and shape of the male-housing receptacle area on the scutal shell plates are positively correlated with the number of males attached (Dreyer et al., Reference Dreyer, Yusa, Gale, Melzer, Yamato and Høeg2018b). Future insights on the aggregation pattern of complemental males on adult hermaphrodites could be done by using a zonal model to determine settlement behaviour and survival of barnacles in relation to distance of the nearest conspecific from a given individual. Future studies should consider barnacle shell isotopes to trace movements and residence time of sea turtles in each habitat, such as has been done for inferring ancient whale migration routes and patterns (Buckeridge et al., Reference Buckeridge, Chan and Lee2018; Taylor et al., Reference Taylor, O'Dea, Bralower and Finnegan2019).
Acknowledgements
The Mabul Sea Turtle Research and Conservation Project headed by P.P. is sanctioned by the Sabah Ministry of Tourism, Culture and Environment, supported with the presence of the Minister during the launch of the fieldwork events, as well as the Sabah Wildlife Department which provided the turtle flipper tags used in this study. The project is also sanctioned by Universiti Malaysia Sabah. We would also like to thank the organizers and all the volunteers of the Mabul sea turtle outreach programme as well as Universiti Malaysia Sabah for assistance in the data collection. Mathan Ganasan is gratefully acknowledged for his contribution towards specimen collection and partial data acquisition. The current study followed rules for ethical treatment of animals as per guidelines recognized by the American Society of Ichthyology and Herpetology (http://www.asih.org/sites/default/files/documents/resources/guidelinesherpsresearch2004.pdf). We thank the reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
Financial support
P.P. received partial funding from Sabah Ministry of Tourism, Culture and Environment (grant no. AM00064) as well as in-kind donations and support from Borneo Divers and Sea Sports (Sabah) Sdn. Bhd and the Mabul World Turtle Day Conservation Fund.













