Abstract
The identification of malnutrition-inflammation-complex (MIC) and functional status (FS) is key to improving patient experience on hemodialysis (HD). We investigate the association of MIC and FS combinations with mortality in HD patients. We analyzed data from 5630 HD patients from 9 countries in DOPPS phases 4–5 (2009–2015) with a median follow-up of 23 [IQR 11, 31] months. MIC was defined as serum albumin < 3.8 g/dL and serum C-reactive protein > 3 mg/L in Japan and > 10 mg/L elsewhere. FS score was defined as the sum of scores from the Katz Index of Independence in Activities of Daily Living and the Lawton-Brody Instrumental Activities of Daily Living Scale. We investigated the association between combinations of MIC (+/−) and FS (low [< 11]/high [≥ 11]) with death. Compared to the reference group (MIC−/high FS), the adjusted hazard ratios [HR (95% CI)] for all-cause mortality were 1.82 (1.49, 2.21) for MIC−/low FS, 1.57 (1.30, 1.89) for MIC+/high FS, and 3.44 (2.80, 4.23) for MIC+/low FS groups. Similar associations were observed with CVD-related and infection-related mortality. The combination of MIC and low FS is a strong predictor of mortality in HD patients. Identification of MIC and poor FS may direct interventions to lessen adverse clinical outcomes in the HD setting.
Similar content being viewed by others
Introduction
The association between measures of protein-energy malnutrition and inflammation in dialysis patients with adverse outcomes is strong1,2,3. Furthermore, it is common for these two conditions to occur concomitantly. Due to these circumstances, researchers have often used the term malnutrition-inflammation complex (MIC) to designate the combination of the two conditions in this population4. Known risk factors for mortality in the general population (e.g., high BMI, high total serum cholesterol) are less frequent in MIC patients and yet they have higher risk of death due to their pro-inflammatory state1.
MIC is also associated with decreased body stores of protein and energy fuels5,6, affecting up to half of hemodialysis (HD) patients7 and ultimately leading to sarcopenia and frailty5,6,8. The initiation of HD is also associated with a decline in functional status (FS) in the elderly9, leading to a vicious cycle of reduced food intake, due to decreased physical function and lack of appetite, resulting in worsened nutritional status1. The Dialysis Outcomes and Practice Patterns Study (DOPPS) showed that low activities of daily living (ADL) is associated with mortality in hemodialysis patients10.
Several national and international guidelines recommend both nutritional and physical therapy for dialysis patients to improve their experience and outcomes11,12,13. Prevention through early identification of patients with MIC, along with the appropriate therapeutic interventions, are important actions to consider in order to improve the patient experience on HD6,14,15.
In this study, we explore the combined effects of MIC and FS on mortality in HD patients.
Results
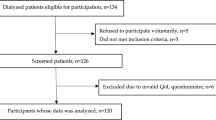
There were 15,266 patients enrolled in DOPPS phases 4 and 5 from facilities that collected CRP routinely in Japan, Europe, and Australia/New Zealand. We excluded patients from facilities that did not often collect albumin (N = 1707). We also excluded patients who did not return a PQ (N = 2968) and those who did not complete the FS assessment (N = 393). Among eligible facilities, we also excluded patients who did not have albumin and/or CRP levels measured at study baseline (N = 500). Our study population consisted of 5630 HD patients with a median follow-up of 23 [IQR 11, 31] months (see STROBE diagram, Fig. 1). Japanese clinics accounted for 42% of patients in our sample, European clinics for 53% and clinics from Australia/New Zealand for 5%. There were 869 deaths over the follow-up period (crude death rate = 8.9 per 100 patient-years), 301 deaths reported with a CV cause (3.1 per 100 patient-years), and 136 deaths due to an infectious cause (1.4 per 100 patient-years).
High CRP was present in 27% of the population (Table 1), ranging from 23% in Japan to 39% in the UK. Low albumin was present in 53% of patients and varied by country: 39% in Spanish patients and as high as 74% of patients in Sweden. MIC was present in 19%, and low FS was observed in 33% of our sample (Table 2). The prevalence of the combinations of MIC (+/−) and FS (low/high) by country is shown in Fig. 2. Japan had the highest prevalence (68%) of patients without any conditions (MIC−/high FS), whereas the prevalence of both (MIC+/low FS) was highest in the UK (17%).
Patient characteristics by MIC and FS combinations are shown in Table 3. Patients in the MIC−/high FS group were much younger (61.1 vs. 71.2), and had lower prevalence of comorbid conditions compared to patients with MIC+ and low FS. The prevalence of heart disease comorbidities was higher in patients who had the combination of MIC+ and low FS, as coronary artery disease was present in 46% of patients with MIC+/low FS and only 25% of patients with MIC−/high FS. Moreover, the prevalence of heart failure was higher in patients with MIC+/low FS (28%) than MIC−/high FS (16%). MIC+/low FS patients also had lower mean creatinine (7.2 mg/dL vs. 9.9 mg/dL), phosphorus values (4.7 vs. 5.2 mg/dL), and a greater use of catheter as vascular access for HD (30% vs. 11%) than MIC−/high FS.
As individual predictors, MIC+ and low FS were each associated with an increased risk of death (HR for MIC+: 2.11 [1.84, 2.42]; HR for low FS: 1.76 [1.50, 2.07]). There was no interaction between MIC and low FS in the association with mortality (p for interaction = 0.40). Estimated effects of MIC/FS combinations on all-cause mortality are provided by level of covariate adjustment in Table 4. The unadjusted associations were very strong (Model 1), and remained robust after adjustment for possible confounders (Model 4). The adjusted hazard ratios (95% CI) for all-cause mortality, compared to the reference group of MIC−/high FS were 1.82 (1.49, 2.21) for MIC−/low FS, 1.57 (1.30, 1.89) for MIC+/high FS, and 3.44 (2.80, 4.23) for MIC+/ low FS.
The cause-specific analysis for CV and infectious mortality followed a similar pattern of association (Table 5), with MIC−/high FS patients having the lowest risk and patients with MIC+/low FS with a nearly four-fold risk for infection-related (HR: 3.91 [2.35, 6.51]) and for CVD-related mortality (HR: 3.97 [2.71, 5.83]). There were no interactions of MIC and FS for CV or infectious mortality (p for interaction = 0.10 and 0.19 for CV and infectious mortality, respectively).
Discussion
This analysis of international DOPPS data shows that MIC in combination with low FS is associated with a high risk of death in HD patients. The presence of one of the components increases the risk by 56% (low FS only) to 75% (MIC+ only). The presence of both conditions results in a more than three-fold higher rate of all-cause mortality, and a nearly four-fold higher rate of CVD- and infection-related death. To the best of our knowledge, this is the first study reporting the combined impact of MIC and low FS in HD patients.
Although there was higher prevalence of diabetes, CVD, and cancer in the MIC+/low FS group, the higher risk of all-cause and cause-specific death persisted even after extensive adjustment for these and other risk factors. Previous observational studies indicated that the inverse association between serum cholesterol and mortality in HD patients, paradoxical in comparison to the general population, is likely due to malnutrition and inflammation5,16. These lines of evidence suggest that malnutrition and systemic inflammation may play important roles in increasing the risk of death in HD patients.
The MIC+/low FS patients had lower mean serum creatinine and phosphorus levels, indicating low muscle mass and protein intake which, along with low FS, represent risk factors for death in dialysis patients17. Interventions to improve nutritional status also improve inflammation, as well as physical performance. A randomized controlled trial (RCT) in which HD patients received oral supplementation with a whey protein, soy protein, or placebo beverage for 6 months before each HD session showed that protein supplementation reduced predialysis interleukin 6 levels and CRP, with gait speed and shuttle walk test improvement observed, as well18. An observational study of an oral nutritional supplement prescription for patients with albumin ≤ 3.5 g/dL during HD showed an association with reduced mortality despite no change in serum albumin levels19. In our study, patients with both MIC+ and low FS had a much greater risk of death due to CV and infection causes than patients with only 1 of the 2 exposures, which is suggestive of a potential biological interaction in the CKD setting.
A systematic review and meta-analysis suggested that physical exercise, including during the HD session, improves physical functioning, cardiovascular fitness, health related quality of life, oxygen uptake (VO2-max), and dialysis adequacy (KT/V) in HD patients20. Furthermore, protein anabolism is enhanced by resistance training before HD and nutrition intake during dialysis21. A RCT showed that a 6-min walking exercise on a treadmill during a dialysis session combined with nutritional supplements is a feasible and well accepted strategy that improves physical function and quality of life22. Therefore, the combined use of nutritional intervention and exercise training may be effective in improving prognosis of MIC and low FS in HD patients such as the ones participating in this study. The Japanese Society of Renal Rehabilitation, as well as other international guidelines, highlight the importance of both nutritional therapy and exercise interventions for patients with PEW11.
DOPPS has shown differences in the mortality rates and practice patterns that affect HD patients’ outcomes between the participating countries23. For instance, the first-year mortality rate in Belgium is greater than in Japan or the United Kingdom23. Our study showed that MIC+/low FS is an independent risk factor for death and was less prevalent in Japan compared to other countries, which might in part explain the lower mortality in Japanese HD patients. On the other hand, similar distributions of MIC+/low FS were found in the United Kingdom and Belgium. DOPPS has also shown that dialysis catheter use is preferred by patients in Belgium, and the rate of catheter use is higher in Belgium than in other countries24. The use of catheters for vascular access in HD is associated with malnutrition and inflammation, as well as a higher risk of death25. Thus, HD-related factors other than MIC+/low FS, such as catheter use, might lead to the difference in mortality across DOPPS countries. However, this analysis was adjusted for catheter use, although residual confounding cannot be completely excluded.
This study has some limitations. Although causal inference cannot be made due to the observational nature of this study, this analysis may be adequate in accomplishing its objective of showing a higher risk of death associated with having these nutritional and inflammation risk factors. We also acknowledge the potential for misclassification of causes of death, which may bias the estimated associations with CVD and infection-related mortality. A clinical trial on MIC/low FS would demand an elaborate intervention that may not have an impact on the outcomes. The use of real-world data from a great number of in-center hemodialysis center patients and standardized protocol for data collection are important strengths of this study. Given the random sampling design of the DOPPS, our study sample can be viewed as representative of the HD population in each participating country, and the large sample size provided sufficient statistical power to detect the proposed associations26. Third, nutrition intake is influenced by food. However, because DOPPS data does not include diet record, we could not evaluate the effects of diet on nutritional status. Fourth, Kanda E. et al. showed the importance and the cutoff levels of serum creatinine levels as a nutritional index using a database of the Japanese Society for Dialysis Therapy8. However, it was difficult to use serum creatinine levels for the categorization of MIC, because three indices might categorize patients into many groups and complicate study design. A simple and accurate index for malnutrition is one of the important themes to improve nutritional conditions of dialysis patients.
Conclusion
This study demonstrates that the combination of MIC and low FS is associated with a higher risk of both all-cause and cause-specific deaths in HD patients. This suggests that the diagnosis of malnourishment and inflammation with albumin and CRP, as well as FS, may be useful for risk stratification for survival in these patients. We believe that this approach will direct attention to nutritional and exercise interventions, based on simple and objective measures, to improve experience and outcomes of patients receiving HD.
Materials and methods
Data source
DOPPS is a prospective cohort study, ongoing since 1996, involving center-based adult chronic HD patients in more than 20 countries. Study sites and patients are randomly selected to achieve national samples. Detail on the study design is included in previous publications16,27 and at http://www.DOPPS.org. DOPPS maintains institutional review board or ethical committee approvals in all participating countries, and informed consent is collected from patients selected for study participation. This work included a cohort of HD patients from Australia, France, Germany, Italy, Japan, New Zealand, Spain, Sweden, and the United Kingdom, enrolled in DOPPS phases 4 (2009–2011) and 5 (2012–2015).
Measures
We defined MIC by the presence of both low albumin and high C-reactive protein (CRP) levels. We considered low albumin as serum albumin levels < 3.8 g/dL. Because CRP levels are systematically lower in Japan, two thresholds for high CRP level were defined: > 3 mg/L in Japan and > 10 mg/L elsewhere28. The distribution of CRP > 3 mg/L in Japan is still lower (prevalence of 23%) than the prevalence of CRP > 10 mg/L in other DOPPS countries (prevalence ranges from 24% in Italy to 39% in the UK).
FS was assessed by the DOPPS self-reported patient questionnaire (PQ), and details were described in the previous study10. In brief, patients indicated their level of ability to perform five activities of daily living tasks (Katz questionnaire: eating, getting dressed, bathing, using the toilet, transferring from bed to chair) and eight instrumental activities of daily living tasks (Brody questionnaire: using the telephone, getting to places beyond walking distance, grocery shopping, preparing meals, doing housework or handyman work, doing laundry, taking medications, and managing money)29,30. Both questionnaires have been validated in the general population. Low FS was defined by a FS score of < 11 out of the 13-points of the combined Katz and Brody scales, calculated as in Jassal et al.10 on which a score of 13 represents full functional independence. Time-to-event outcomes included all-cause mortality (primary) and cause-specific death (secondary) due to (1) CVD or (2) infection. We explored both the main effects and the combined effect between the two exposure variables on mortality.
Patient/study sample
Patients were included if they answered all 13 FS questions on the PQ and had available data on serum albumin and CRP in the three months before PQ completion. We limited our sample to facilities that routinely measured CRP and albumin levels in over half of their patients to limit cases where they were measured “by indication”. We excluded patients with a prior history of limb amputation to accurately evaluate BMI.
Statistical analyses
Cox proportional hazards models were used to analyze the association of the combinations of MIC (+/−) and FS (low/high) with mortality, stratified by country and DOPPS phase, and accounting for facility clustering using robust sandwich covariance estimators. Models were adjusted for case-mix (patient characteristics: age, sex, body mass index, vintage, and comorbid history of diabetes mellitus, hypertension, coronary artery disease, congestive heart failure, other heart diseases, cerebrovascular disease, cancer, gastrointestinal bleeding, psychiatric disorder, peripheral vascular disease, recurrent cellulitis, and lung disease), central venous catheter use, and laboratory values (serum creatinine and phosphorus levels, white blood cell count [WBC], and hemoglobin level). Time at risk started at the completion of the PQ and ended at the time of death, 7 days after leaving the facility due to transplant or transfer, 7 days after changing modality, loss to follow-up, or administrative study end. The proportional hazards assumption was confirmed. Additionally, cause-specific Cox models were used to test the association of MIC and/or FS with cause-specific (infection, CVD-related) mortality. Deaths by other causes were censored in this approach.
Overall, missingness for covariates was low (< 20% for the majority of covariates). For missing data, we used the sequential regression multiple imputation method implemented by IVEware31, and the MIAnalyze procedure in SAS/STAT 9.4. All analyses were carried out using SAS software, version 9.4 (SAS institute, Cary, NC).
Data availability
The data that support the findings of this study are available from Arbor Research Collaborative for Health, but restrictions apply to the availability of these data which were used for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Arbor Research Collaborative for Health.
References
Kalantar-Zadeh, K., Ikizler, T. A., Block, G., Avram, M. M. & Kopple, J. D. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am. J. Kidney Dis. 42(5), 864–881 (2003).
Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 19(1), 53–61 (2001).
Kopple, J. D. McCollum Award Lecture, 1996: protein-energy malnutrition in maintenance dialysis patients. Am. J. Clin. Nutr. 65(5), 1544–1557 (1997).
Stenvinkel, P., Heimbürger, O., Lindholm, B., Kaysen, G. A. & Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 15(7), 953–960 (2000).
Fouque, D. et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 73(4), 391–398 (2008).
Ikizler, T. A. et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 84(6), 1096–1107 (2013).
Carrero, J. J. et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J. Ren. Nutr. 28(6), 380–392 (2018).
Kanda, E., Kato, A., Masakane, I. & Kanno, Y. A new nutritional risk index for predicting mortality in hemodialysis patients: nationwide cohort study. PLoS ONE 14(3), e0214524 (2019).
Kurella Tamura, M. et al. Functional status of elderly adults before and after initiation of dialysis. N. Engl. J. Med. 361(16), 1539–1547 (2009).
Jassal, S. V. et al. Functional dependence and mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 67(2), 283–292 (2016).
Yamagata, K. et al. Clinical practice guideline for renal rehabilitation: systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Renal Replacement Therapy 5(28), 1–19 (2019).
Ashby, D. et al. Renal association clinical practice guideline on haemodialysis. BMC Nephrol. 20(1), 379 (2019).
ERBP: Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher. http://www.european-renal-best-practice.org/sites/default/files/u33/English%20-%20Elderly%20summary%20OK%20print.pdf (Accessed 15/07/2020)
Liu, Y. et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 291(4), 451–459 (2004).
Kopple, J. D., Zhu, X., Lew, N. L. & Lowrie, E. G. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 56(3), 1136–1148 (1999).
Pisoni, R. L. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am. J. Kidney Dis. 44(5 Suppl 2), 7–15 (2004).
Kalantar-Zadeh, K., Block, G., McAllister, C. J., Humphreys, M. H. & Kopple, J. D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 80(2), 299–307 (2004).
Tomayko, E. J., Kistler, B. M., Fitschen, P. J. & Wilund, K. R. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J. Ren. Nutr. 25(3), 276–283 (2015).
Weiner, D. E. et al. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am. J. Kidney Dis. 63(2), 276–285 (2014).
Heiwe, S. & Jacobson, S. H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am. J. Kidney Dis. 64(3), 383–393 (2014).
Majchrzak, K. M., Pupim, L. B., Flakoll, P. J. & Ikizler, T. A. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol. Dial. Transplant. 23(4), 1362–1369 (2008).
Hristea, D. et al. Combining intra-dialytic exercise and nutritional supplementation in malnourished older haemodialysis patients: towards better quality of life and autonomy. Nephrology (Carlton). 21(9), 785–790 (2016).
Robinson, B. M. et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 388(10041), 294–306 (2016).
Fissell, R. B. et al. Hemodialysis patient preference for type of vascular access: variation and predictors across countries in the DOPPS. J. Vasc. Access. 14(3), 264–272 (2013).
Hung, A. M. & Ikizler, T. A. Hemodialysis central venous catheters as a source of inflammation and its implications. Semin. Dial. 21(5), 401–404 (2008).
Wasserstein, R. L. & Lazar, N. A. The ASA’s statement on p-values: context, process, and purpose. Am. Stat. 70, 129–133 (2016).
Young, E. W. et al. The Dialysis Outcomes and Practice Patterns Study: an international hemodialysis study. Kidney Int. 57(Suppl 74), S74–S81 (2000).
Bazeley, J. et al. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 6(10), 2452–2461 (2011).
Katz, S., Downs, T. D., Cash, H. R. & Grotz, R. C. Progress in development of the index of ADL. Gerontologist. 10(1), 20–30 (1970).
Lawton, M. P. & Brody, E. M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9(3), 179–186 (1969).
Raghunathan ,T.E., Solenberger, P.W., Berglund, J., Van Hoewyk, J. IVEware: Imputation and variance estimation software. Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan, https://www.src.isr.umich.edu/software/. (2002).
Acknowledgements
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial support. Figures were created by Lopes and Karaboyas in SAS software, version 9.4 and MS PowerPoint. The DOPPS is led by the following investigators in each participating country: Ali Alaradi (Bahrain); Pieter Evenepoel, Michel Jadoul (Belgium); Manish Sood, Rita Suri (Canada); Xiaonong Chen, Yuqing Chen, Fanfan Hou, Xinling Liang, Zhaohui Ni, Li Zuo (China); Christian Combe, Fitsum Guebre-Egziabher, Pablo Antonio Ureña Torres (France); Werner Kleophas, Elke Schaeffner, Thomas Weinreich (Germany); Giuliano Brunori, Loreto Gesualdo, Francesco Locatelli, (Italy); Masafumi Fukagawa, Masaaki Inaba, Masaomi Nangaku, Kosaku Nitta, Kazuhiko Tsuruya (Japan); Saeed Al-Ghamdi, Mohammed Al Ghonaim, Fayez Hejaili, Ayman Karkar, Faissal Shaheen, Jamal Al Wakeel (Kingdom of Saudi Arabia); Naser Alkandari, Anas Alyousef, Bassam Al Helal (Kuwait); Issa Alsalmi, Yacoub Al Maimani (Oman); Fadwa Al Ali, Abdulla Hamad (Qatar); Aleix Cases, Almudena Vega Martínez, Patricia de Sequera (Spain); Anders Christensson, Stefan Jacobson (Sweden); Samra Abouchacra, Mohamed Hassan, Ali Abdulkarim Al Obaidli, Mona Al Rukhaimi, Abdul Kareem Saleh (United Arab Emirates); Elham Asgari, Indranil Dasgupta, Hugh Rayner (United Kingdom). Country Investigators from prior DOPPS study phases can be found at: https://www.dopps.org/OurStudies/HemodialysisDOPPS.aspx.
Funding
Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. This manuscript was directly supported by Kyowa Kirin Co., Ltd.
Author information
Authors and Affiliations
Contributions
(1) Substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data: I.D., A.K., E.K., K.I., K.T., M.B.L., S.J., B.B., B.M.R., H.H. (2) Drafting the article or revising it critically for important intellectual content: I.D., A.K., E.K., K.I., M.B.L., S.J., B.B., B.M.R. (3) Final approval of the version to be published: I.D., A.K., E.K., K.I., K.T., M.B.L., S.J., B.B., B.M.R., H.H.
Corresponding author
Ethics declarations
Competing interests
Kazuhiko Tsuruya has received honoraria from Chugai, Kyowa Kirin, Astellas, Mitsubishi Tanabe, Bayer, Kissei, Sumitomo Dainippon, Torii, and Baxter; has received consulting fee from Chugai and Astellas; has received grants from Baxter, Torii, Chugai, and Kyowa Kirin. Eiichiro Kanda, Kunitoshi Iseki, Hideki Hirakata, Stefan H Jacobson, and Indranil Dasgupta have no conflicts of interest to declare.Marcelo Barreto Lopes, Angelo Karaboyas, Brian Bieber, and Bruce M. Robinson are employees of Arbor Research Collaborative for Health, which administers the DOPPS.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanda, E., Lopes, M.B., Tsuruya, K. et al. The combination of malnutrition-inflammation and functional status limitations is associated with mortality in hemodialysis patients. Sci Rep 11, 1582 (2021). https://doi.org/10.1038/s41598-020-80716-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80716-0
This article is cited by
-
The impact of malnutrition on the effectiveness of intradialytic exercise in hemodialysis patients: amulticenter cohort study
International Urology and Nephrology (2024)
-
Evaluation of laboratory values affecting mortality of end-stage renal disease patients: a competing risks approach
BMC Nephrology (2023)
-
Prognostic value of creatinine-to-cystatin c ratio in patients with type 2 diabetes mellitus: a cohort study
Diabetology & Metabolic Syndrome (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.